Download Proteins Misfolding Disease and more Lecture notes Biochemistry in PDF only on Docsity!
Protein-Misfolding Diseases
PHY
April 8 th 2009
Jessica Nasica
Overview of the presentation
- (^) General facts on protein folding
- (^) Causes of misfolding and protein aggregation
- (^) Cellular consequences of protein aggregation
- (^) Protein-misfolding diseases
- (^) Amyloidoses
- (^) Amyloid fibril formation
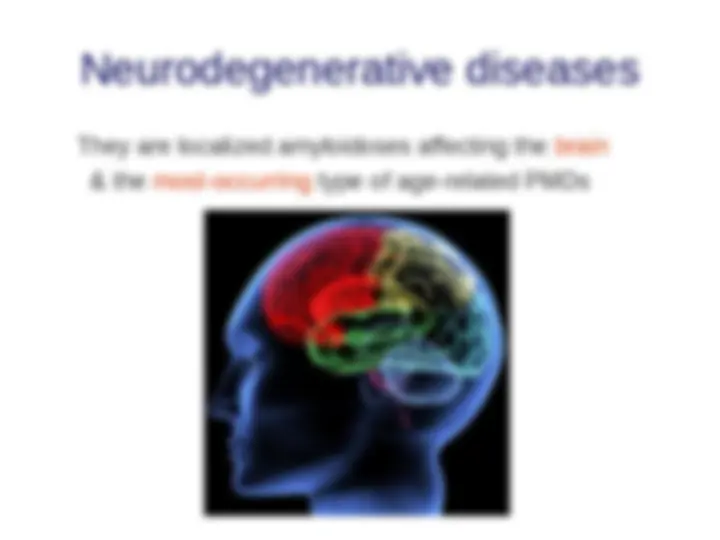
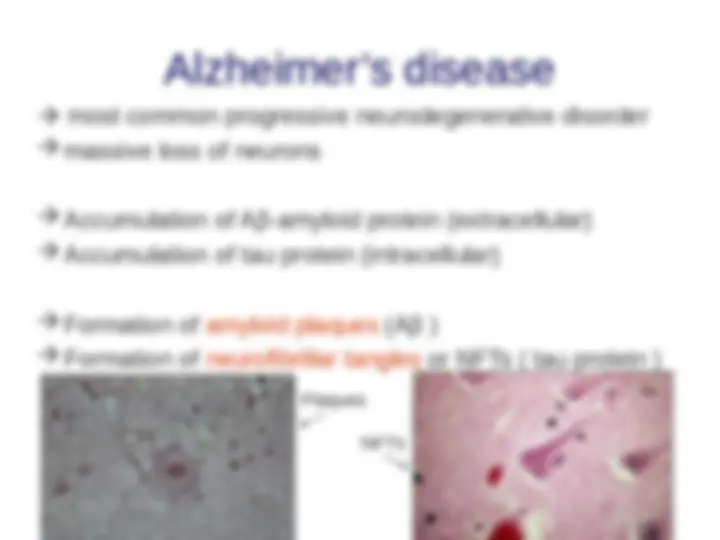
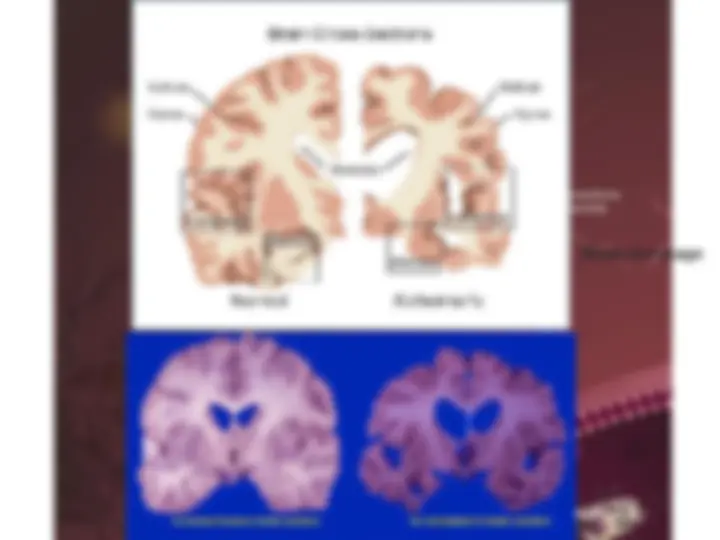
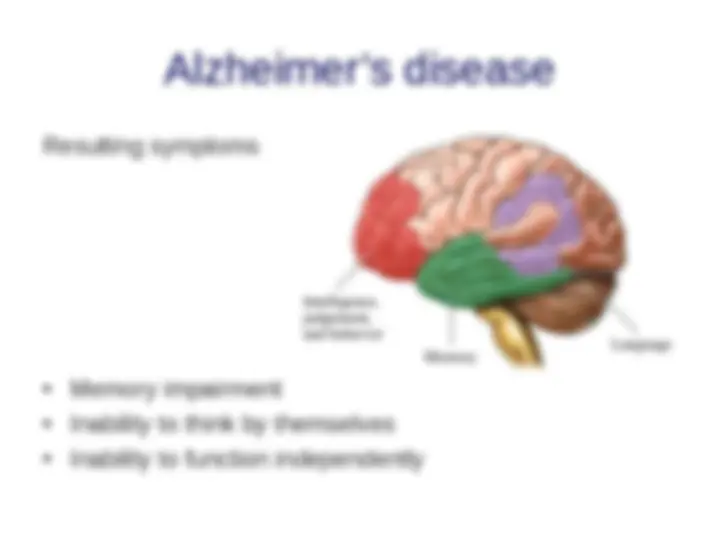
- (^) Neurodegenerative diseases and Alzheimer’s disease
- (^) Therapy solutions
- (^) Curing Alzheimer’s disease
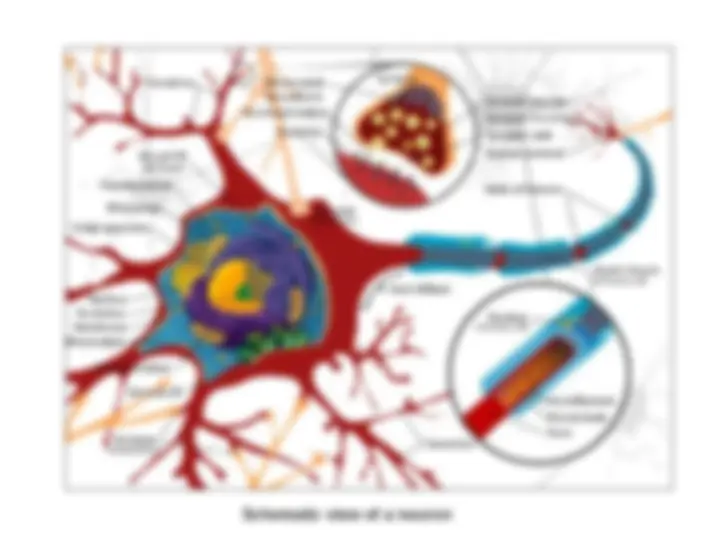
General facts on protein folding
The “new view”
- (^) A stochastic search of the many conformations accessible to one particular sequence
- (^) A polypeptide chain searches for the lowest-energy state by a process of trial and error
- (^) Native-like interactions between residues are more stable than non-native ones
- (^) To undergo correct folding, the native structure must go through a transition state Possible starting configurations : 10^ Compact configurations : 10^ Transition states : 10^ Native conformation : 1 Lowest energy state only 1 amino acid sequence
General facts on protein folding
The Protein Quality Control (PQC) system
found in ER and cytosol
General facts on protein folding
Chaperones
The concentration of chaperones is genetically self- regulated and increases with the presence of misfolded proteins. They possess an ATPase domain that reversibly binds with the hydrophobic parts of partially folded proteins
General facts on protein folding
• Important elements for protein folding:
- (^) The amino acid sequence
- (^) The right cellular environment
- (^) The perfect balance between the various folding states
- (^) A fully functional Protein Quality Control (PQC) system (T ,P ,pH , etc …) (Chaperones, proteasome unit )
Causes of misfolding and protein aggregation How protein aggregates form
- (^) Change in cellular conditions more misfolded proteins the PQC system is overwhelmed -> aggregation is favored
- (^) Aggregation is thought to be set in by protein segments containing hydrophobic amino acids residues, β-sheet predisposition and low net charge.
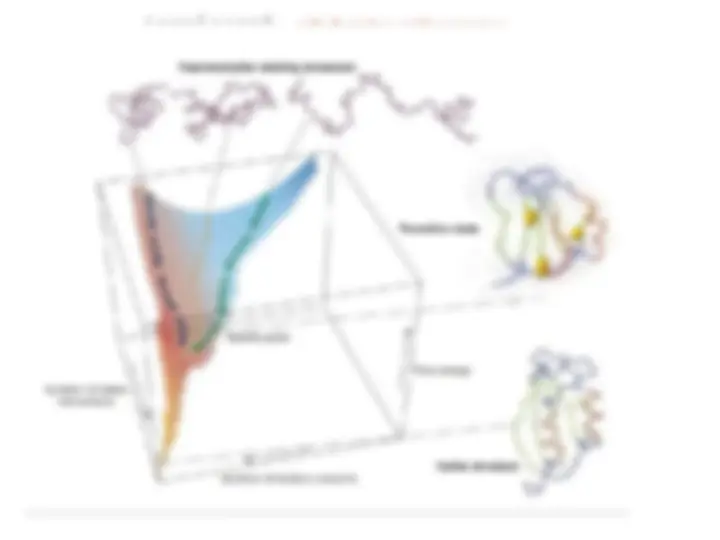
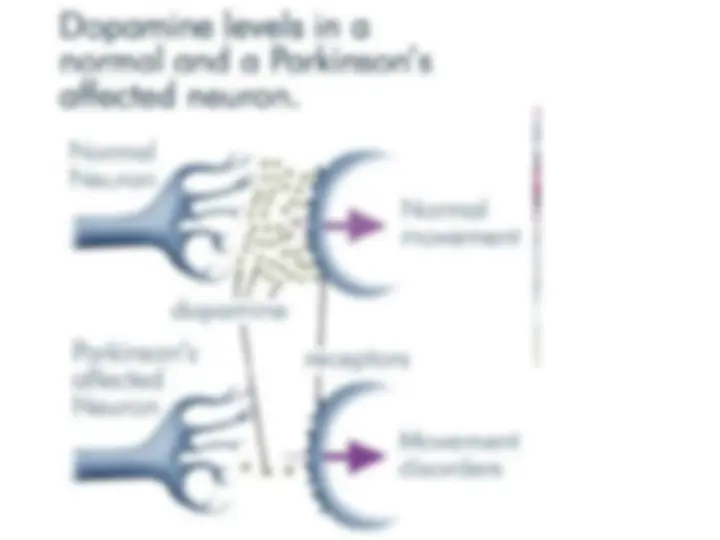
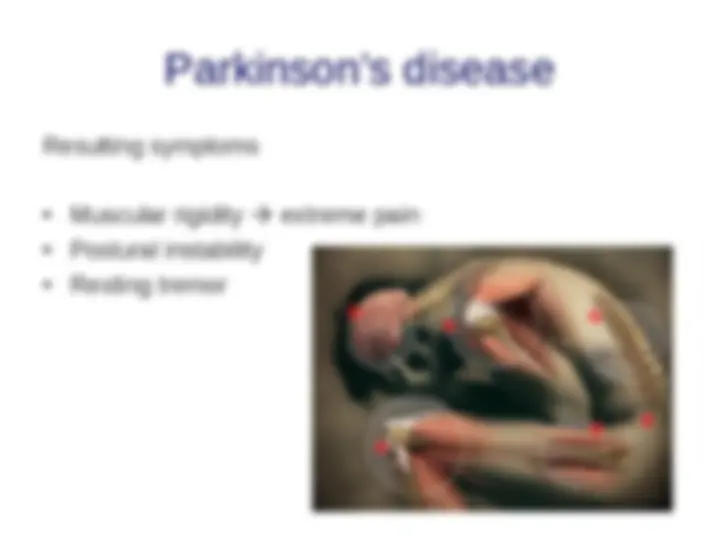
Causes of misfolding and protein aggregation States accessible to a protein molecule Free-synuclein gene mutations (Parkinson’s)energy folding landscape for chaperone-synuclein gene mutations (Parkinson’s)mediated protein folding
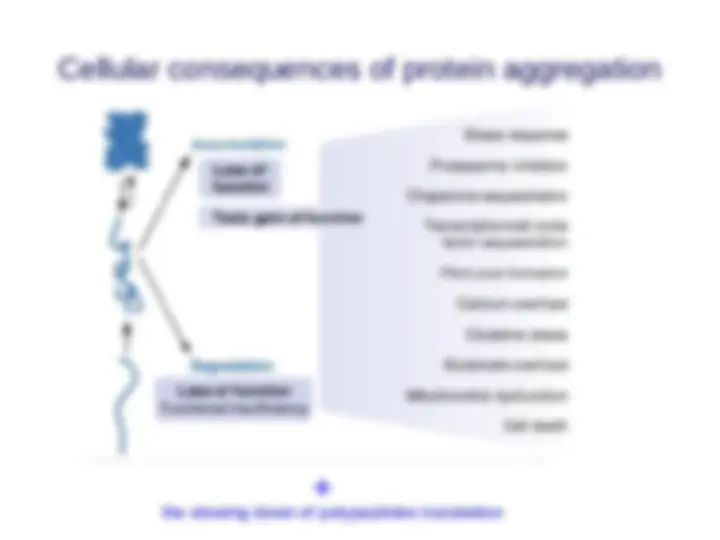
Cellular consequences of protein aggregation
- (^) Loss-of-function pathogenesis: if misfolded proteins are prematurely degraded by PQC system protein deficiency disease
- (^) Gain-of-function pathogenesis: if misfolded proteins are not eliminated but accumulated instead disease pathology toxicity Some diseases display both pathogenic mechanisms.
Cellular consequences of protein aggregation the slowing down of polypeptides translation +
Protein-misfolding diseases
Protein-misfolding diseases
Conditions may be :
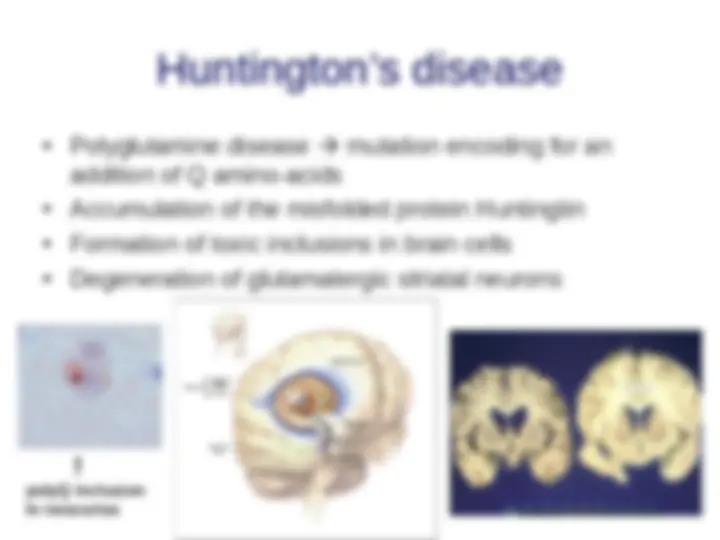
- (^) Familial the disorder is genetically inherited and symptoms appear during childhood ( e.g., Huntington )
- (^) Sporadic patternless and characterized by a late onset. Primarily due to aging or to an incorrect lifestyle. Not associated with gene mutations. (e.g., most of Alzheimer’s and Parkinson’s cases and many more)
- (^) Transmissible (e.g., prion disease, spongiform encephalopathies and fatal familial insomnia)
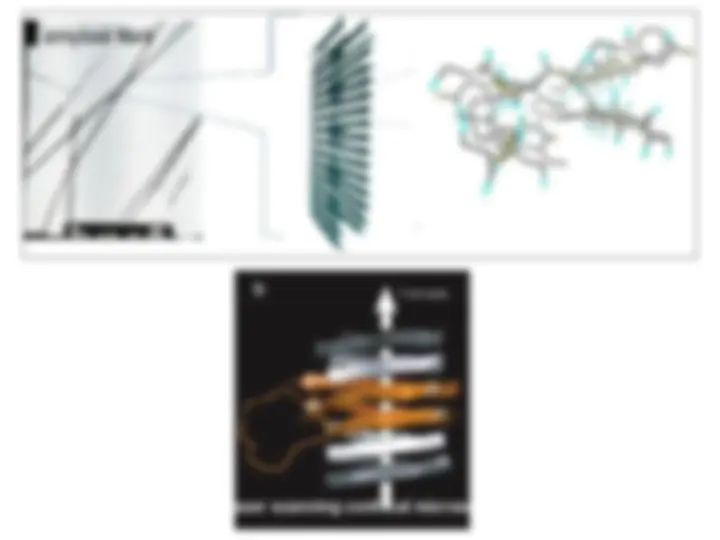
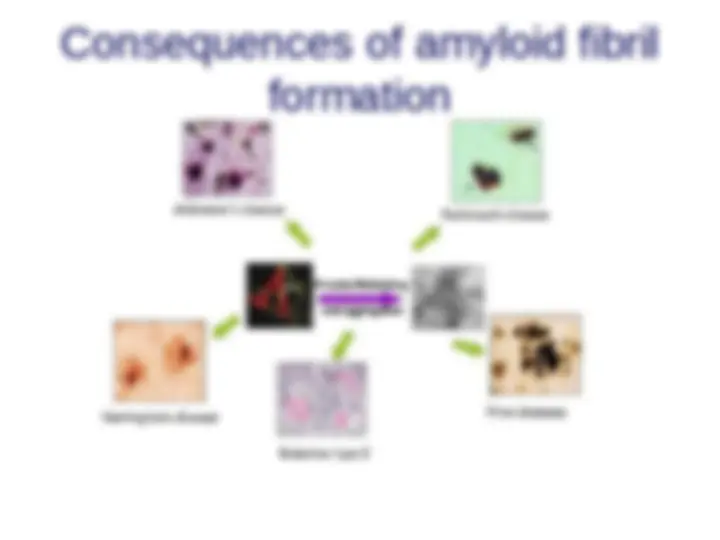
A closer look at amyloidoses
Characterized by the formation, accumulation and deposition of similar highly-organized insoluble fibrillar aggregates amyloid fibrils AFM image of β2-synuclein gene mutations (Parkinson’s)microglobulin amyloid fibrils
Causes of the formation of amyloid fibrils