





























































































Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Data on the photosynthetic and transpiration characteristics of Acacia Koa leaves and phyllodes. The study compares various factors such as CO2 exchange rates, chlorophyll content, total soluble protein, and RuBPcase+ between leaves and phyllodes. The document also discusses the impact of light intensity and orientation on these characteristics.
Typology: Exercises
1 / 128

This page cannot be seen from the preview
Don't miss anything!





























































































Gerald Alan Walters
Dissertation Committee: Duane P. Bartholomew, Chairman James A. Silva Peter P. Rotar Paul C. Ekern Douglas J. C. Friend
We certify that we have read this dissertation and that in our opinion it is satisfactory in scope and quality as a dissertation for the degree of Doctor of Philosophy in Agronomy and Soil Science.
DISSERTATION COMMITTEE
Acacia koa Gray is Hawaii's most valuable forest tree. It is considered a pioneering species because it becomes established rapidly after overstory removal if seeds are present in the soil. The two leaf forms of koa apparently allow it to gain and then maintain control of the site. Leaves and phyllodes of koa differ markedly in leaf orientation, morphology, and anatomy, and in the levels of chlorophyll, total soluble protein, and Ribulose-l,5-bisphosphate carboxylase. Both leaf forms have similar CO^-exchange rates when determined on a leaf area basis. Mean maximum rates of photosynthesis
and light compensation for both leaf forms occurred at 1200 and 25
about 55 ppm for both leaves and phyllodes, indicating that both leaf forms fix C 0 2 via the C^-pathway. On an organ basis leaves have higher CX^-exchange rates than phyllodes. Leaves also have higher transpiration rates than phyllodes. Growth and development of koa seedlings was greatly affected by available light. Seedlings grown under the highest light levels were the tallest and had the largest stem diameter and total dry weight. All measured growth parameters decreased as light intensity decreased. As the seedlings grew and developed under the different light levels, roots as a percentage of the total dry weight remained a fairly constant 18 percent. Initially, leaves made up more than 50 percent of the total dry weight. The percentage of leaf dry weight
iv
decreased with time, while the percentage of stem dry weight increased. Phyllodes developed only on seedlings exposed to light of at least light-saturating levels. The data indicated that koa seedlings can survive and grow only at light levels equal to or greater than 25 percent of full sunlight. The vigor of koa seedlings grown at less than 25 percent full sunlight declined with time and it appeared that they would eventually die. This minimum light requirement accounts for the scarcity of natural koa reproduction in an undisturbed koa forest. Koa leaves and phyllodes readily adapted to changes in available light. Leaves and phyllodes grown in full sunlight developed the characteristics of sun leaves. Conversely, at 27 percent of full sunlight, both leaf forms developed the characteristics of shade leaves. Fully-developed leaves and phyllodes only adapted physiologically to changes in available light. Partially-developed leaves and phyllodes adapted both physiologically and anatomically to changes in available light. Seedlings with phyllodes grown in 27 percent of full sunlight for 6 to 8 weeks developed leaves at the terminals. Seedlings again produced phyllodes when placed in full sunlight.
TABLE OF CONTENTS (Continued) Page APPENDIX A Solar Radiation, Cumulative Rainfall, and Temperatures at Waimanalo, Hawaii for the Period October 1, 1980 to April 28, 1981 ......... 104 LITERATURE CITED ................................................... 105
vii
Representative Transpiration Rates for Crop Plants and Trees ........................ Morphological Characteristics of Leaves and Phyllodes of Acacia Koa .................. Stomatal Frequency and Pore Length in Acacia Koa Leaves and Phyllodes ............. CC^-Exchange Rate by a Single Fully- expandedBased on AcaciaProjected koa LeafLeaf Areaand Phyllode,.................. Chlorophyll in Leaves and Phyllodes of Acacia Koa ...................................... Total Soluble Protein and RuBPcase+ in Leaves and Phyllodes of Acacia koa ........... Transpiration by a Single Fully-expanded Acacia koa Leaf and Phyllode, Based on Projected Unit and Organ Area ................ Total Leaf Conductance of a Single Fully- expanded Acacia koa Leaf and Phyllode Based on Projected Leaf Area .................. Photosynthetic Rates of Acacia koa Leaves and Phyllodes Expressed on the Basis of Leaf Area, Chlorophyll, and RuBPcase+.... Calculated Boundary Layer conductance of a Hypothetical A. Koa Leaflet and Phyllode at Different Wind Speeds ...................... Stem, Leaf, and Root Development of Acacia Koa Seedlings Grown Under Different Light Levels. Dry Weight Distribution in Stems, Leaves, and Roots of Acacia Koa Seedlings Grown Under Different Light Levels .................. Growth Analysis Components for Acacia Koa Seedlings Grown Under Different Light Levels.
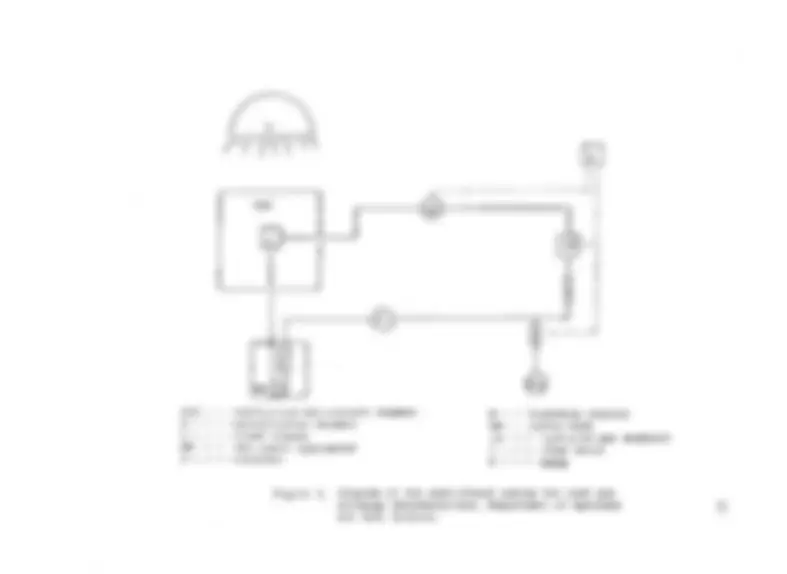
Figure Page 1 Acaciaand b) KoaP h y l l o d e s Seedling ........................................ with a) Leaves 4 2 An Assimilation Acacia Koa ChamberPhyllode forin Gasthe Leaf Exchange Determinations ............................... 26 3 Diagram of the Semi-closed System for Leaf Gas Exchange Determinations, Department of Agronomy and Soil S c i e n c e ............. 27 4 Semi-closed system for leaf gas exchange determinations, Department of Agronomy and Soil S c i e n c e ........................... 28 5 Cross Section of a) Leaflet (210X) and b) Phyllode (105X) of Acacia K o a ....................... 34
6 Rate of ( ^ - e x c h a n g e as a Function of Flux Incident Density Photosynthetic of Leaves and PhotonPhyllodes of Acacia K o a .......................................... 36 7 Rate of CC^-exchange as a Function of Incident Photosynthetic Photon Flux Density of Acacia Koa Phyllodes in Vertical and Flat P o s i t i o n s ......................... 39 8 Dry Weight Increments of Stem (A ), Leaves (*), Roots (□), and Total (+) of Acacia Koa Seedlings Grown Under Different Light Treatments ............................. 65
Koa (Acacia koa Gray) has been called the Monarch of Hawaiian forests. This is a fitting description of this native leguminous species that occurs on all the larger islands, forming a part of the forest cover on about 500,000 acres (Nelson and Wheeler 1963), or about 25 percent of the forested areas of the state. Koa, one of the most common native tree species of the Hawaiian Island ecosystem, provides a habitat for several species of native birds (Goodwin and Aldrich 1966, Munro 1960), mollusks (Kondo 1970), and insects (Gagne et al. 1970, Gressitt and Davis 1969, Swezey 1925). Many native plant species grow in association with koa. Koa is also a renewable economic resource and is Hawaii's most valuable timber tree. Skolmen (1970) estimated that the koa industry generates more than $1.3 million per year. Koa grows to heights of more than 45 m. The circumference of one tree measured 8.4 m (Littlecott 1969). The technical wood properties of koa are almost identical to those of black walnut (Juglans nigra L.) (Skolmen 1968) and the beauty of finished koa and black walnut are comparable. Koa has many uses, but because of its high value, it is used mainly for cabinets, furniture, gun stocks, veneer, and craft pieces. Koa forests are not as extensive today as they once were (Hall 1904, Whitesell 1964) and the area continues to dwindle for a number of reasons. In the past 50 years, an estimated 100,000 acres of koa forest have been cleared for pasture. Koa is relished by cattle who prevent the regeneration of koa forests by eating all seedlings and
\
regeneration has been accomplished by direct seeding (Bryan 1929, Whitesell 1967) and with planting (Walters 1974, Walters and Horiuchi 1979). The successful and rapid establishment of a koa forest by natural or artificial means requires much information about the effects of environmental factors on the growth of koa seedlings. The climatic requirements of koa are not well known. Koa is found at elevations ranging from about 180 m (Rock 1920) to 2100 m (Judd 1920), but reaches its greatest development in size and number at elevations of 1500 to 1800 m. The mean January temperature at these elevations is about 12 o^ C; the mean August temperature is 15 o C. Although koa occurs in semiarid to wet areas, it attains its greatest development in areas that receive 1900 to 5000 mm of rain annually (Whitesell 1964). Irradiance in the koa forest varies from full sunlight in
Active Radiation (PAR) 400-700 nm] at 1500-m elevation, to perhaps less than one-tenth full sunlight under a forest canopy. Koa seedlings are heterophyllous. They initially bear leaves that are finely divided, consisting of five to seven pairs of pinnae, each pinna with 12 to 24 pairs of leaflets. These true leaves give way to smooth, stiff crescent-shaped "leaves" which are in fact expanded petioles called phyllodes (Neal 1965, Rock 1920) (Fig. 1). During the transition from true leaves (juvenile form) to phyllodes (mature form) (Hartmann and Kester 1975), it is common to see true leaves attached to the phyllodes. Generally, mature trees bear only phyllodes. However, if the trunk is wounded, the sprouts that often
Figure 1. Acacia koa seedling with a) leaves and b) phyllodes.
Despite the economic and ecological importance of koa, data describing its growth and development in the nursery and field and the physiological biochemical data necessary to explain that growth and development, are lacking. Because 90 to 95 percent of the dry weight of plants are derived from photosynthetic C 0 2 assimilation (Zelitch 1975a), an understanding of the partitioning of photosynthate into various plant parts is necessary. Some of the CC>2 fixed during photosynthesis is utilized by respiration in the light and darkness to produce energy and products that are used in the maintenance and construction of plant tissue (Ledig et al. 1976). The rate of photosynthesis is often related to water deficits in the plant. As deficits occur because moisture loss by transpiration exceeds uptake, cell elongation rates decline and eventually photosynthetic rates decrease because of increased stomatal and mesophyll diffusive resistances (Begg and Turner 1976, Zelitch 1975b). This general literature review will focus on the processes of photosynthesis, respiration, transpiration, and their subsequent effects on plant growth and development. Because of the lack of information on Acacia species in general, and koa specifically, the review includes information on other tree and plant species. Carbon Dioxide Exchange of Leaves Photosynthesis is a photochemical reaction in which water plus carbon dioxide (C02) in the presence of light and chlorophyll yields sugar. The overall reaction of photosynthesis is given by the
simplified equation: nCO (^) ^ + 2nH 0* + light chloroplasts (CH (^) ^0) n + nO (^) z* + nfl 0^ The initial phase of photosynthesis is the trapping of light energy by chlorophyll contained in plant organs, principally the leaves. During this phase, electrons are removed from water, oxygen is released, and high energy pyridine nuceleotide and adenosine triphosphate molecules are produced. These energy-rich molecules are used to reduce carbon dioxide during the second phase of photosynthesis as well as to provide energy for other physiological processes (Zelitch 1971). This energy-trapping process of the initial phase is apparently the same for all higher plants, but the manner in which the plant reduces carbon dioxide varies with species (Govindjee and Govindjee 1974). All woody plants and trees examined thus far, with the exception of mangrove (Joshi et al. 1974) and larch (Fry and Phillips 1976), have characteristics of plants (Downton 1971). Classification as a plant implies the absence of a highly photosynthetically active vascular bundle sheath in the leaf tissue, the potential of high rates of photorespiration, the predominance of ribulose-l,5-bisphosphate carboxylase/oxygenase (RuBPcase) as the major photosynthetic C 0 2 fixation enzyme, and a minimum CO^ compensation concentration of about 50 ppm C 0 2 (Black 1973, Zelitch 1971). Photorespiration reduces net photosynthesis by releasing part of the C 0 2 fixed in photosynthesis with a loss of energy associated with its fixation (Black et al. 1976, Chollet and Ogren 1975, Goldsworthy 1970, Schrader 1976, Zelitch 1975a). The high CO^ compensation concentration
photosynthesis were considerably higher in the sun species (16-20 mg C O 2 dm-2 h-1 ) than in the shade species (2-5 mg C O 2 dm-2 h-1 ). The light-compensation point is the PPFD at which the rate of C O 2 release is equal to COj-uptake at a constant C O 2 concentration and some physiological temperature. This point is a useful indicator of the maintenance requirement of plants at low PPFD (Schaedle 1975). Characteristically, shade-adapted leaves have a much lower light- compensation point than light-adapted leaves (Loach 1967, Schaedle 1975). The shade and sun species examined by Bohning and Burnside (1956) had light-compensation points of 2 and 20 umol m -2^ s -1, respectively. Differences in light-compensation points are caused primarily by differences in respiration rates. When the respiration rate is low, the leaf requires less PPFD to photosynthesize rapidly enough to balance the C 0 2 being lost, so the light-compensation point is also low (Salisbury and Ross 1978). The C O 2 -compensation point is the C O 2 concentration where photosynthetic fixation just balances respiratory loss. The C 0 2 ~compensation point provides an indication of the pathway by which CC> 2 is fixed in the leaf. A C02_comP ensati°n point between 0 to 5 ppm indicates the C-4 pathway, whereas a C O 2 -compensation point between 50 to 100 ppm indicates the C-3 pathway (Singh et al. 1974). Leaves and other structures capable of assimilating C O 2 follow a developmental progression from the time they are initiated and this progression influences the O ^ - e x c h a n g e rate (CER) (Shibles 1976). Differences in CER are attributable to related morphological,
physiological, and biochemical events.i The pattern of leaf development is similar for different species (Schaedle 1975). During leaf expansion, increases in photosynthetic CO^-uptake can be related to development of internal leaf structure and stomates (Homann 1975, Isebrands and Larson 1973), a decrease in diffusion resistance (Homann 1975) , synthesis of chlorophyll (Dickmann 1971b), development of physiological and structural integrity of the membrane-bound phosphorylation system of chloroplasts (Dickmann 1971b, Hernadez-Gil and Schaedle 1973), Fraction I protein synthesis, increases in RuBPcase activity (Dickmann 1971b), and a sharp decline in mitochondrial respiration (Dickmann et al. 1975). A leaf is mature when leaf expansion ceases and leaf anatomy has essentially stabilized. Maturation does not proceed at a uniform rate throughout the leaf. The lamina tip matures first, both structurally and functionally, and maturation then proceeds basipetally, the leaf base and margins maturing last (Isebrands and Larson 1973). At maturity, mesophyll cells and intercellular spaces are fully developed, stomatal formation is complete, and the leaf vascular system is fully functional (Isebrands and Larson 1973). A leaf that has reached anatomical maturity is functionally mature as well (Dickmann et al. 1975). The potential capacity for net photosynthesis is maximum and COj-compensation concentration and dark respiration reach a minimum (Dickmann 1971a, Dickmann and Gjerstad 1973, Larson et al. 1969, Loach and Little 1973). The Hill reaction and RuBPcase activities are maximum (Dickmann et al. 1975, Ghosh 1973). A mature leaf functions only as an exporter of photosynthate (Larson et al.