







Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
An in-depth analysis of various enzyme-catalyzed reactions, focusing on mono-oxygenases, di-oxygenases, oxidases, and peroxidases. These enzymes play a crucial role in oxygenation reactions, where molecular oxygen is transferred into organic acceptor molecules. The mechanisms of these enzymes, including cofactor recycling, and provides examples of their applications in biotransformations.
Typology: Slides
1 / 12

This page cannot be seen from the preview
Don't miss anything!







2
Some example of monooxygenase catalyzed biotransformations
2
Docsity.com
Sub
Sub
S b
Catalytic cycle of cytochrome P-450 dependent monooxygenases
Fe
Fe
Fe
Sub
2
SubO O^2
Fe
3+ N
3+ N
Fe
5+ N
Sub
Sub
Sub
2
Fe S N
Fe S N
Fe S N
Sub O4+Fe^ S N N
Asymmetric microbial hydroxylation by monooxygenase of iso-butyric acid
microorganismO
2
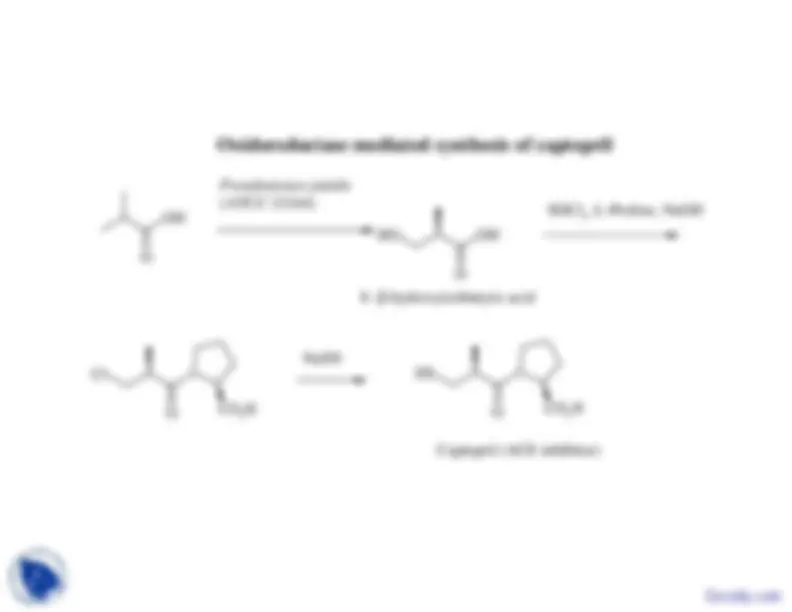
Oxidoreductase mediated synthesis of captopril
The use of substrate engineering in bihydroxylation
The
use of substrate engineering in bihydroxylation