







Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Organic chemistry in medicine lectures
Typology: Lecture notes
1 / 11

This page cannot be seen from the preview
Don't miss anything!







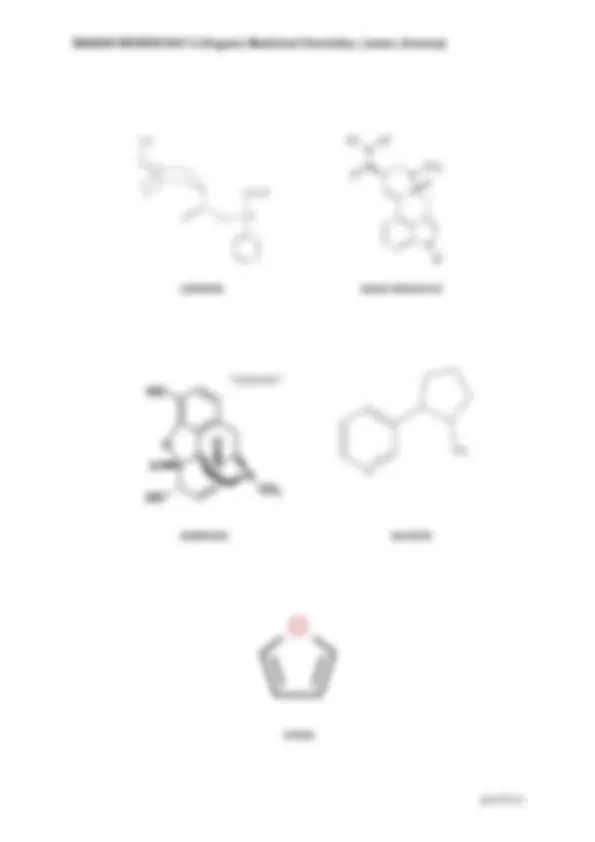
Indole Indoline Pyrrolidine VINCRISTINE Indole ring (double bond in the 5-membered ring containing nitrogen) Indoline- no double bond BLEOMYCIN Pyrimidine- a ring structure composed of 4 carbon atoms and 2 nitrogen atoms (meta position) Pyrazine- a ring structure containing 4 atoms of carbon and 2 of nitrogen (para position) Pyranose- 6 - membered ring system consisting of 5 carbon atoms with 1 oxygen atom Furanose- 5 - membered ring consisting of 4 carbon atoms with 1 oxygen atom Thiazole- a ring structure composed of 3 carbon atoms, 1 nitrogen atom, and 1 sulfur atom Imidazole- a ring structure composed of 3 carbon atoms and 2 nitrogen atoms at nonadjacent positions Pyrimidine Imidazole Thiazole Pyranose
Quinoline Pyridine Piperidine DRUG METABOLISM: Phase 1 metabolites – POLAR but retain some non-polarity character therefore they are able to cross cellular membranes than phase 2. (Phase 2 are much polar; exceptions: Acetylated and Methylated metabolites) Least likely excretion product of Aspirin: Glycine conjugate (carboxylic acid) Ester glucuronide (carboxylic acid) Unchanged drug (aspirin is metabolized in the body) Hydroxylated metabolite (aromatic ring system; Ester undergoes hydrolysis) Carboxylic acid Aromatic Ring Ester *hydroxylation – attachment of OH in the para position Glucuronysyltransferase – resp for the glucuronidation phase 2 rxn (infants are not capable of glucuronidation bec the enzyme is still underdeveloped; gray baby syndrome – chloramphenicol) N- acetyltransferase – acetylation (phase 2) Azoreductase= Azoreduction (phase 1) – nitrogen double bonded w another nitrogen Methyltransferase – methylation (phase 2) NOT A THERAPEUTIC ADV OF THE USE OF PRODRUG: POTENCY *Prodrug- once metabolized, it’ll become active, potency will not change whatever is the active form of the prodrug Therapeutic advantages of prodrugs: § Oral absorption § Water sol § Duration of action ORAL (PO) ROUTE- subject to first-pass metab in the liver Terms used to describe the metabolic reaction:
Benzodiazepine Barbiturate (Clonazepam) Aryloxypropanolamine (Atenolol) *beta blockers- chem classifies as aryloxypropanolamine ESSENTIAL FOR THE CHOLINERGIC ACT: § Ethylene bridge § Quaternary amine § Ester fxnl grp STABILITY AGAINST HYDROLYSIS: Addition of Methyl group to provide steric hindrance: Carbamates – stable to hydrolysis (carbachol) Replacement of ester grp w carbamate grp: Prevents hydrolysis of acetoxy portion (Methacholine) *Replacement of ester with ether group (results to loss of activity) PETIT MAL/ Absence seizure (DOC: ethoxusimide – chem class: Succimides) Hydantoin – chem class of phenytoin Alpha blocker that does not contain quinazoline structure: TAMSULOSIN (ONLY non-quinazoline alpha blocker) TAMSULOSIN: used for BPH (Benign prostatic hyperplasia) *ZOSINs are alpha blockers with quinazoline (Prazosin, Terazosin, Alfuzosin, etc) Phenylalkylamine – verapamil Benzothiazepine – dialthiazem Dihydropyridine – nifedipine (all ends with dipine; CCBs) Sulfamoylanthranilic acid – Furosemide Benzothiazide – HCTZ NaOH + Methenamine = formaldehyde *Methenamine is said to be converted to formaldehyde in the urinary tract (acts as urinary antiseptic) *Methenamine acts as a prodrug of formaldehyde Selective toxicity – prop of chemicals to destroy one form of life w/ o harming other A SUBSTANCE IS CLASSIFIED AS AN ANTIBIOTIC IF:
Sulbactam – ampicillin Robert Koch – germ theory; father of modern bacteriology Methenamine (Urotropin) § Treat UTI where it is degraded in acidic urine, liberating formaldehyde as the active agent § Should be administered w a urinary acidifier to activate the drug Urinary acidifiers: Ammonium chloride; Monobasic sodium phosphate Fxnl group resp. for the toxicity of chloramphenicol: NITRO (metabolized via reduction= produces amine to prevent its toxicity) Examples of naturally- occurring methylxanthines: § Theophylline – 1, 3 - dimethylxanthine § Theobromine – 3, 7 - dimethylxanthine § Caffeine – 1, 3, 7 - trimethylxantine AMPHETAMINE: not naturally-occuring *hydrogenase – belong to CYP 450 CYP – 450 - most impt component of the mixed fxn oxidase system Binding of drugs to serum proteins like albumin: PROTEIN BINDING Hydrolysis – cleavage of all molecules by the use of water Proteolysis – breakdown of proteins and peptides into amino acids Biotransformation – metabolism DRUG- RECEPTOR COMPLEX – formed when drug was combined to a receptor in order to elicit pharmacologic response Antiseptics – prevent infection by the destruction of pathogenic microorganism when applied to LIVING TISSUES Disinfectants - prevent infection by the destruction of pathogenic microorganism when applied to INANIMATE OBJECTS MECHANISMS OF ANTIMICROBIAL ACTION: § Halogenation – halogens (ex. Chlorine and bromine) § Oxidation – oxidizing agents (ex. Hydrogen peroxide) § Protein precipitation – heavy metals (ex. Silver) Gentamicin is isolated from: Micromonospora purpurea Aztreonam: Chromobacterium violaceum Mupirocin: Pseudomonas fluorescens 4 - aminoquinoline: CHLOROQUINE and AMOQUINOLINE 8 - aminoquinoline: PRIMAQUINE, QUINOLINE METHANOLS, QUININE SULFONAMIDE NITROGEN (NHR^2 ): § Either Primary or Secondary What will happen if you remove the oxygen bridge of the drug? NO CHANGE. *MORPHINANS – series of compounds that was produced when the oxygen bridge is removed (ex. Levorphanol) Analog of Adenosine: DIDANOSINE(ddI): deoxyadenosine; I: INOSINE (precursor of adenosine) Thymidine analog: § Stavudine (d4T)
§ Zidovudine (AZT) Cytosine analog: § Lamivudine (3TC) § Zalcitabine (ddC) *3rd letter will tell the analog type. *Exception GUANOSINE ANALOG: Abacavir (ABC) walang kinalaman yung letter C Demeclocycline: INTERMEDIATE ACTING TETRACYCLINE Oxytetracycline: SHORT ACTING Tigecycline: VERY LONG ACTING Minocycline and Doxycycline: LONG ACTING Transition state inhibitors: § Zanamivir (^) Neuraminidase inhibitor § (^) Oseltamivir § (^) Aliskiren – renin inhibitor DOC for acute attack of PLASMODIUM VIVAX – CHLOROQUINE Radical cure for malaria – PRIMAQUINE Drug that has LITTLE to NO sedative qualities: ASTEMIZOLE (2nd^ gen antihistamine) *first gen antihistamines: more sedating § Tripelennamine § Diphenhydramine § Promethazine Example of Androgen: FLUOXYMESTERONE Megestrol acetate: Progesterone Quinestrol (Ester of Ethinyl Estradiol) and Ethinyl Estradiol: (Estrogen derivative) Prednisone: GLUCOCORTICOID AROMATASE INHIBITORS: § Exemestane – steroidal § Anastrozole – non- steroidal § Letrozole – non- steroidal Steroidal aromatase Inhibitor: EXEMESTANE Non- steroidal: ANASTROZOLE and LETROZOLE (Triazole Non-steroidal aromatase inhibitors) What do sulfonamides mimic when acting as enzyme inhibitors? PABA (Para-aminobenzoic acid) 2 nd^ gen anti- psychotics: § Clozapine § Loxapine § Aripiprazole § Quetiapine Pentazocine: BENZOMORPHAN (always end with “zocinE”) Alfentanil: Phenylpiperidines (“fentanil” sa name) Levorphanol/ Levomorphan: Morphinans