

















Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
It's an MCAT study guide 2022.
Typology: Study Guides, Projects, Research
1 / 24

This page cannot be seen from the preview
Don't miss anything!

















Human Memory
Sensory Memory
Short-term Memory
Long-term Memory (< 1 sec)
Explicit Memory (conscious)
Declarative Memory (facts, events)
Episodic Memory (events, experiences)
Semantic Memory (facts, concepts)
Procedural Memory (skills, tasks)
Implicit Memory (unconscious)
(< 1 min) (lifetime)
Working Memory
Problem-solving techniques include trial-and- error , algorithms , deductive reasoning (deriving conclusions from general rules) and inductive reasoning (deriving generalizations from evidence). Heuristics (simplified principles used to make decisions, “rules of thumb”), biases, intuition, and emotions may assist decision-making, but may also lead to erroneous or problematic decisions.
Motivation is the purpose or driving force behind our actions.
Motivation theories
Weak Low High Arousal
Optimal arousal Optimal performance
Impaired performance because of strong anxiety
Increasing attention and interest
Performance
MotIvAtIon, EMotIon, AnD StrESS
Stage EEg Waves features Awake Beta and alpha
Able to perceive, process, access, and express information 1 Theta Light sleep 2 Theta Sleep spindles and K complexes 3/4 Delta Slow-wave sleep; dreams; declarative memory consolidation; some sleep disorders rEM Mostly beta
Appears awake physiologically; dreams; paralyzed; procedural memory consolidation; some sleep disorders Sleep disorders include dyssomnias (amount or timing of sleep), such as insomnia, narcolepsy, sleep apnea, and sleep deprivation; and parasomnias (odd behaviors during sleep), such as night terrors and sleepwalking (somnambulism).
Drug addiction is mediated by the mesolimbic pathway , which includes the nucleus accumbens, medial forebrain bundle , and ventral tegmental area. Dopamine is the main neurotransmitter.
Drug group function Depressants (alcohol, barbiturates, benzodiazepines)
Sense of relaxation and reduced anxiety
Stimulants (amphetamines, cocaine, ecstasy)
Increased arousal
opiates/opioids (heroin, morphine, opium, pain pills)
Decreased reaction to pain; euphoria
hallucinogens (LSD, peyote, mescaline, ketamine, psilocybin- containing mushrooms)
Distortions of reality and fantasy; introspection
Marijuana has some features of depressants, stimulants, and hallucinogens (in very high doses).
CognItIon, ConSCIouSnESS, AnD lAnguAgE
UCS (food) UCR No Response
Neutral stimulus (bell)
CR (salivation)
CS (bell)
UCS (food) UCR No Response
Neutral stimulus (bell)
CR (salivation)
CS (bell)
Negative reinforcement
Positive reinforcement
Behavior
Continues
Stops
Positive punishment
Negative punishment
lEArnIng AnD MEMorY
Somatic symptom and related disorders
Personality disorders
Patterns of inflexible, maladaptive behavior that cause distress or impaired functioning
SoCIAl thInkIng
Social stratification is based on socioeconomic status (SES).
Incidence:
new cases population at risk
per time
Prevalence:
number of cases(new or old)
total population
per time
Morbidity: the burden or degree of illness associated with a given disease Mortality: deaths caused by a given disease
SoCIAl StrAtIfICAtIon
-
SoCIAl IntErACtIon
Demographics: the statistical arm of sociology Migration refers to the movement of people into (immigration) or out of (emigration) a geographical location. Demographic transition: a model used to represent drops in birth and death rates as a result of industrialization
SoCIAl StruCturE AnD DEMogrAPhICS
SoCIAl ProCESSES, AttItuDES, AnD BEhAvIor
Focuses on the tendency for individuals to infer the causes of other people’s behavior
SoCIAl PErCEPtIon AnD BEhAvIor
Once replicated, the bacterial cells can be used to create a protein of interest, or can be lysed to allow for isolation of the fragment of interest from the vector.
DNA libraries are large collections of known DNA sequences.
Hybridization is the joining of complementary base pair sequences.
Polymerase chain reaction ( PCR ) is an automated process by which millions of copies of a DNA sequence can be created from a very small sample by hybridization.
DNA molecules can be separated by size using agarose gel electrophoresis.
Southern blotting can be used to detect the presence and quantity of various DNA strands in a sample. After electrophoresis, the sample is transferred to a membrane that can be probed with single-stranded DNA molecules to look for a sequence of interest.
DNA sequencing uses dideoxyribonucleotides , which terminate the DNA chain because they lack a 3 ' –OH group.
Central Dogma: DNA → RNA → proteins
Degenerate code allows multiple codons to encode for the same amino acid.
Point mutations can cause:
RNA is structurally similar to DNA except:
There are three major types of RNA in transcription:
RNA AND THE GENETIC CODE
Figure I-3-4. Expression of a Prokaryotic Protein Coding Gene
3 ' untranslated region (UTR)
5 ' 3 '
5 ' 3 '
3 ' 5 '
5 ' UTR 3 ' UTR
–10 coding region
transcription
promoter
TGA
transcription
translation
ATG
coding region
UGA GC-rich stem AUG and loop
transcription terminates
H 2 N–protein–COOH
5 ' untranslated region (UTR)
TATA box
UUUUUU
Shine–Dalgarno sequence
DNA
mRNA
Steps:
Occurs at the ribosome.
U A C
Met
A U G C C G U A U G C U
P site A site
U A C
Met
G G C
Pro
A U G C C G U A U G C U
G G C Pro incoming tRNA^ }
anticodon
Three stages: initiation , elongation , termination Posttranslational modifications:
Operons (Jacob–Monod model) are inducible or repressible clusters of genes transcribed as a single mRNA.
inducible system repressible system
regulator
RNA polymerase
promoter operator structural
repressor I I R
R
R
repressor inducer—repressor complex cannot bindto operator—structural genes are transcribed
inducer
binds R R C RC
regulator
repressor
corepressor (end product)
repressor—corepressorcomplex binds to operator and repressesenzyme synthesis repressor cannotbind to operator by itself
RNA polymerase
promoter operator structural
Transcription factors search for promoter and enhancer regions in the DNA.
Osmotic pressure , a colligative property , is the pressure applied to a pure solvent to prevent osmosis and is related to the concentration of the solution. Π = iM R T Passive transport does not require ATP because the molecule is moving down its concentration gradient or from an area of higher concentration to an area of lower concentration.
molecules (^) transport proteins
cell membrane
concentration gradient simple diffusion facilitated diffusion passive transport active transport Figure 1.5 Movement Across Memberances
energy (ATP or ion gradient)
Endocytosis and exocytosis are methods of engulfing material into cells or releasing material to the exterior of cells, both via the cell membrane. Pinocytosis is the ingestion of liquid into the cell from vesicles formed from the cell membrane and phagocytosis is the ingestion of solid material.
BIOLOGICAL MEMBRANES
Occurs in the cytoplasm of all cells, and does not require oxygen. Yields 2 ATP per glucose. Important enzymes include:
CARBOHYDRATE METABOLISM
Takes place in mitochondrial matrix. Main purpose is to oxidize acetyl-CoA to CO 2 and generate high- energy electron carriers (NADH and FADH 2 ) and GTP.
Figure I-13-1. Citric Acid Cycle
Fatty acids Ketones Alcohol
Citrate
Acetyl-CoA
Pyruvate Glucose Amino acids PDH
synthaseCitrate
cis-Aconitase Isocitrate
α-Ketoglutarate α -Ketoglutarate dehydrogenase Succinyl-CoA
NAD+ NAD+
NAD+
GTP
FADH (^2) FAD
Fumarate
Fumarase
Malate
Malate dehydrogenase
GDP + Pi
NADH
NADH
NADH
CO (^2)
Succinyl-CoA synthetase
Succinate
Succinate dehydrogenase (complex II)
Oxaloacetate
Isocitrate dehydrogenase CO 2
Takes place on the matrix-facing surface of the inner mitochondrial membrane. NADH donates electrons to the chain, which are passed from one complex to the next. Reduction potentials increase down the chain, until the electrons end up on oxygen, which has the highest reduction potential.
Complex I Complex II Complex III Complex IV
centers^ Fe-S 4 H+^ 2 H+ FMN NADH NAD+^ + H +
2 e –
QH^ Q 2 QH 2 2 H + Heme
Fe
FAD FADH 2 Succinate Fumarate + 2 H +
centersFe-S
2 H+
2 H+
Cyt c(ox) Cyt c(red) Q Q
Cyt c(ox) Cyt c(red) Q Q
Step 1 Step 2 1 e – 1 e –
1 e – 1 e –
4 × 4 ×
2 H+
2 H+
4 H+^ + O 2
Fe Fe
Cu Cu
Cyt c(ox)
Cyt c(red) 4 e –
2 H 2 O
2 H+ Q QH (^2) Q QHQH (^2 )
NADH cannot cross the inner mitochondrial membrane, so must use one of two shuttle mechanisms to transfer its electrons to energy carriers in the mitochondrial matrix: the glycerol 3-phosphate shuttle or the malate–aspartate shuttle.
The proton-motive force is the electrochemical gradient generated by the electron transport chain across the inner mitochondrial membrane. The intermembrane space has a higher concentration of protons than the matrix; this gradient stores energy, which can be used to form ATP via chemiosmotic coupling. ATP synthase is the enzyme responsible for generating ATP from ADP and an inorganic phosphate (Pi ). Summary of the energy yield of the various carbohydrate metabolism processes:
Glycogenesis (glycogen synthesis) is the building of glycogen using two main enzymes:
Occurs in both the cytoplasm and mitochondria, predominantly in the liver. Most of gluconeogenesis is just the reverse of glycolysis, using the same enzymes. The three irreversible steps of glycolysis must be bypassed by different enzymes:
Occurs in the cytoplasm of most cells, generating NADPH and sugars for biosynthesis. Rate-limiting enzyme is glucose-6-phosphate dehydrogenase , which is activated by NADP+^ and inhibited by NADPH and insulin.
Lipids are transported via chylomicrons , VLDL , IDL , LDL , and HDL.
TGLCE
Liver
Adipose
Cholesterol
Chylomicron(lymph) Chylomicron(blood)
Chylomicronremnant
Triacylglycerol Glucose
Fatty acids
Triacylglycerol
Glycerol 3-P
Glycerol 3-P
Fatty acids
TGLCE
IDL
(blood)VLDL
TGLchol
TGLCE
TGLchol
(epithelium)^ Intestine Lipoproteinlipase
Lipoproteinlipase
Protein digestion occurs primarily in the small intestine. Carbon skeletons of amino acids are used for energy, either through gluconeogenesis or ketone body formation. Amino groups are fed into the urea cycle for excretion.
LIPID AND AMINO ACID METABOLISM
Insulin Glucagon
Gluconeogenesis
Glycogenolysis Lipolysis Protein catabolism Ureagenesis
Glycogensynthesis
synthesisProtein
synthesisLipid
Cellular glucoseuptake
Glucose efflux Ketogenesis
Plasma glucose
utilizationGlucose
Stimulates Inhibits
BIOENERGETICS AND REGULATION OF METABOLISM
Direct hormones directly stimulate organs; tropic hormones stimulate other glands. Mechanisms of hormone action: peptides act via second messengers and steroids act via hormone/receptor binding to DNA. Amino acid-derivative hormones may do either.
Ectoderm “Attract”oderm
Nervous system, epidermis, lens of eye, inner ear Endoderm “Endernal” organs
Lining of digestive tract, lungs, liver and pancreas Mesoderm “Means”oderm
Muscles, skeleton, circulatory system, gonads, kidney
I Rest All gates closed II Depolarization Na gates open+ III Repolarization Na gates inactivate+ K gates open+ IV Hyperpolarization All gates closed
Blood type
RBC antigen
Antibodies Donates to:
Receives From: A A anti-B A, AB A, O B B anti-A B, AB B, O AB A, B None AB only All O None anti-A, B All O only
Enzyme Production Site Function Site Function Pepsin Gastric glands(chief cells) Stomach Hydrolyzes specific peptide bonds
Trypsin Pancreas Small Intestine
Hydrolyzes specific peptide bonds Converts chymotrypsinogen to chymotrypsin Chymotrypsin Hydrolyzes specific peptide bonds Carboxypeptidases A and B Hydrolyzes terminal peptide bond at C-terminus Aminopeptidase Intestinal glands
Hydrolyzes terminal peptide bond at N-terminus Dipeptidases Hydrolyzes pairs of amino acids Enteropeptidase Converts trypsinogen to trypsin
Table 1 Surface Colony Growth Starch Digestion A B C A B C Strain 1 + + + – – – Strain 2 + + – + + – key: + = growth; – = no growth
Table 2 Surface Colony Growth Deep-Agar Colony Growth Strain 1 + – Strain 2 + + key: + = growth; – = no growth
Hormone Source Action F ollicle-stimulating (FSH)
Anterior pituitary
Stimulates follicle maturation; spermatogenesis L uteinizing (LH) Stimulates ovulation; testosterone synthesis A drenocorticotropic (ACTH) Stimulates adrenal cortex to make and secrete glucocorticoids T hyroid-stimulating (TSH) Stimulates the thyroid to produce thyroid hormones P rolactin Stimulates milk production and secretion E ndorphins Inhibits the perception of pain in the brain G rowth hormone Stimulates bone and muscle growth/lipolysis Oxytocin Hypothalamus; stored in posterior pituitary
Stimulates uterine contractions during labor, milk secretion during lactation Antidiuretic (ADH, vasopressin) Stimulates water reabsorption in kidneys Thyroid hormones (T 3 , T 4 ) (^) Thyroid Stimulates metabolic activity Calcitonin Decreases (tones down) blood calcium level Parathyroid hormone Parathyroid Increases blood calcium level Glucocorticoids (^) Adrenal cortex Increases blood glucose level and decreasesprotein synthesis; anti-inflammatory Mineralocorticoids Increases water reabsorption in kidneys Epinephrine, Norepinephrine Adrenal medulla Increases blood glucose level and heart rate
Glucagon Pancreas
Stimulates conversion of glycogen to glucose in the liver; increases blood glucose Insulin (^) Lowers blood glucose; increases glycogen stores Somatostatin Supresses secretion of glucagon and insulin Testosterone Testes Maintains male secondary sexual characteristics Estrogen (^) Ovary/Placenta Maintains female secondary sexual characteristics Progesterone Promotes growth/maintenance of endometrium Melatonin Pineal Regulates sleep–wake cycles Atrial natriuretic peptide Heart Involved in osmoregulation and vasodilation Thymosin Thymus Stimulates T-cell development
Enzyme Production Site Function Site Salivary amylase (ptyalin) Salivary glands^ Mouth Pancreatic amylase Pancreas Small intestine Maltase Intestinal glands Small intestine Sucrase Intestinal glands Small intestine Lactase Intestinal glands Small intestine
Hydrolysis Reaction
Starch → maltose Starch → maltose Maltose → 2 glucoses Sucrose → glucose, fructose Lactose → glucose, galactose
ENDOCRINE SYSTEM
The functional unit is the neuron: cell body
dendrites
Schwann cells nodes ofRanvier myelinsheath axon (^) nerve terminals
Ectoderm “Attract”oderm
Nervous system, epidermis, lens of eye, inner ear Endoderm “Endernal” organs
Lining of digestive tract, lungs, liver and pancreas Mesoderm “Means”oderm
Muscles, skeleton, circulatory system, gonads, kidney
I Rest All gates closed II Depolarization Na gates open+ III Repolarization Na gates inactivate+ K gates open+ IV Hyperpolarization All gates closed
Blood type
RBC antigen
Antibodies Donates to:
Receives From: A A anti-B A, AB A, O B B anti-A B, AB B, O AB A, B None AB only All O None anti-A, B All O only
Enzyme Production Site Function Site Function Pepsin Gastric glands(chief cells) Stomach Hydrolyzes specific peptide bonds
Trypsin Pancreas Small Intestine
Hydrolyzes specific peptide bonds Converts chymotrypsinogen to chymotrypsin Chymotrypsin Hydrolyzes specific peptide bonds Carboxypeptidases A and B Hydrolyzes terminal peptide bond at C-terminus Aminopeptidase Intestinal glands
Hydrolyzes terminal peptide bond at N-terminus Dipeptidases Hydrolyzes pairs of amino acids Enteropeptidase Converts trypsinogen to trypsin
Hormone Source Action F ollicle-stimulating (FSH)
Anterior pituitary
Stimulates follicle maturation; spermatogenesis L uteinizing (LH) Stimulates ovulation; testosterone synthesis A drenocorticotropic (ACTH) Stimulates adrenal cortex to make and secreteglucocorticoids
T hyroid-stimulating (TSH) Stimulates the thyroid to produce thyroid hormones P rolactin Stimulates milk production and secretion E ndorphins Inhibits the perception of pain in the brain G rowth hormone Stimulates bone and muscle growth/lipolysis Oxytocin Hypothalamus; stored in posterior pituitary
Stimulates uterine contractions during labor, milk secretion during lactation Antidiuretic (ADH, vasopressin) Stimulates water reabsorption in kidneys Thyroid hormones (T 3 , T 4 ) (^) Thyroid Stimulates metabolic activity Calcitonin Decreases (tones down) blood calcium level Parathyroid hormone Parathyroid Increases blood calcium level Glucocorticoids (^) Adrenal cortex Increases blood glucose level and decreasesprotein synthesis; anti-inflammatory Mineralocorticoids Increases water reabsorption in kidneys Epinephrine, Norepinephrine Adrenal medulla Increases blood glucose level and heart rate
Glucagon Pancreas
Stimulates conversion of glycogen to glucose in the liver; increases blood glucose Insulin (^) Lowers blood glucose; increases glycogen stores Somatostatin Supresses secretion of glucagon and insulin Testosterone Testes Maintains male secondary sexual characteristics Estrogen (^) Ovary/Placenta Maintains female secondary sexual characteristics Progesterone Promotes growth/maintenance of endometrium Melatonin Pineal Regulates sleep–wake cycles Atrial natriuretic peptide Heart Involved in osmoregulation and vasodilation Thymosin Thymus Stimulates T-cell development
Enzyme Production Site Function Site Salivary amylase (ptyalin) Salivary glands^ Mouth Pancreatic amylase Pancreas Small intestine Maltase Intestinal glands Small intestine Sucrase Intestinal glands Small intestine Lactase Intestinal glands Small intestine
Hydrolysis Reaction
Starch → maltose Starch → maltose Maltose → 2 glucoses Sucrose → glucose, fructose Lactose → glucose, galactose
action potential
action potential
action potential
- - - - - - - - - - - - -
+ + + + + +
- – + + + + + + - + Na+ **+
K +
K +
**+
+**
+ +
- - - - - - - **-
+ + – – + + + +
+
- **Na+
K +
K +
**+
+**
+ +
+ +
+ +
**- – – –
-**
+ +
+ + + + – –
+
- **Na+
axon
NERVOUS SYSTEM
FSH
follicle begins to mature estrogen uterus vascularization of uterine wall
LH surge follicle^ ruptures-egg released(ovulation) corpus luteum pr ogesterone
pr egnancy corpus luteum atrophies(inhibition stops, cycle starts anew)
day 0
ovary
pituitary (prevents multiple egg development)
day 14
early in cycle later in cycle
LH
uterine wallmaintains
no pregnancy
zygote hCG (LH analog)
Initiation:
tropomyosin
actin filament (^) troponin
calcium myosin binding site
Relaxation:
MUSCULOSKELETAL SYSTEM
pulmonary veins L. pulmonary artery
R. pulmonary artery
aorta
superior vena cava inferior vena cava
R. atrium
L. atrium mitral valve L. ventricle
tricuspid ValveR. ventricleseptum
Superior and inferior vena cava → right atrium → right ventricle → pulmonary arteries → lungs→ pulmonary veins → left atrium → left ventricle → aorta → body Three portal systems: Blood travels through an extra capillary bed before returning to the heart.
Plasma: aqueous mix of nutrients, wastes, hormones, blood proteins, gases, and salts Erythrocytes (red blood cells): carry oxygen
Leukocytes (white blood cells): function in immunity Platelets: clotting
Antigens are located on the surface of red blood cells.
Ectoderm “Attract”oderm
Nervous system, epidermis, lens of eye, inner ear Endoderm “Endernal” organs
Lining of digestive tract, lungs, liver and pancreas Mesoderm “Means”oderm
Muscles, skeleton, circulatory system, gonads, kidney
I Rest All gates closed II Depolarization Na gates open+ III Repolarization Na gates inactivate+ K gates open+ IV Hyperpolarization All gates closed
Blood type
RBC antigen
Antibodies Donates to:
Receives From: A A anti-B A, AB A, O B B anti-A B, AB B, O AB A, B None AB only All O None anti-A, B All O only
Hormone Source Action imulating (FSH)
Anterior pituitary
Stimulates follicle maturation; spermatogenesis g (LH) Stimulates ovulation; testosterone synthesis rticotropic (ACTH) Stimulates adrenal cortex to make and secrete glucocorticoids timulating (TSH) Stimulates the thyroid to produce thyroid hormones Stimulates milk production and secretion ns Inhibits the perception of pain in the brain ormone Stimulates bone and muscle growth/lipolysis
Hypothalamus; stored in posterior pituitary
Stimulates uterine contractions during labor, milk secretion during lactation ic (ADH, sin) Stimulates water reabsorption in kidneys ormones (T 3 , T 4 ) (^) Thyroid Stimulates metabolic activity Decreases (tones down) blood calcium level id hormone Parathyroid Increases blood calcium level ticoids (^) Adrenal cortex Increases blood glucose level and decreasesprotein synthesis; anti-inflammatory orticoids Increases water reabsorption in kidneys
ine, Norepinephrine Adrenal medulla Increases blood glucose level and heart rate
Pancreas
Stimulates conversion of glycogen to glucose in the liver; increases blood glucose Lowers blood glucose; increases glycogen stores atin Supresses secretion of glucagon and insulin one Testes Maintains male secondary sexual characteristics
rone Ovary/Placenta^ Maintains female secondary sexual characteristicsPromotes growth/maintenance of endometrium Pineal Regulates sleep–wake cycles iuretic peptide Heart Involved in osmoregulation and vasodilation Thymus Stimulates T-cell development
Production Site Function Site
mylase (^) Salivary glands Mouth
amylase Pancreas Small intestine Intestinal glands Small intestine
Hydrolysis Reaction
Starch → maltose Starch → maltose Maltose → 2 glucoses
Blood cells with Rh factor are Rh+; these individuals produce no anti-Rh antibody. Rh –^ blood cells lack the antigen; these individuals produce an antibody if exposed.
CIRCULATION
0
20
20
40
40
60
60
80
80
100
100
% saturation of hemoglobin
P (^) O 2 (mmHg)
35 25 curve shifts to the RIGHT pH shifts DOWN
RESPIRATION
diaphragm alveoli
trachea
bronchus
bronchiole
B-lymphocytes memory cells remember antigen, speed up secondary response
plasma cells make and release antibodies ( IgG , IgA , IgM , IgD , IgE ), which induce antigen phagocytosis
T-lymphocytes cytotoxic T-cells destroy cells directly helper T-cells activate B- and T-cells and macrophages by secreting lymphokines
suppressor T-cells regulate B- and T-cells to decrease anti-antigen activity
memory cells
Includes skin, passages lined with cilia, macrophages, inflammatory response, and interferons (proteins that help prevent the spread of a virus)
IMMUNE SYSTEM
small intestine
colon
pancreas
stomach
esophagus
anus rectum
duodenum
gallbladder
liver
pharynx
trachea
tongue
oral cavity
DIGESTION
Ectoderm “Attract”oderm
Nervous system, epidermis, lens of eye, inner ear Endoderm “Endernal” organs
Lining of digestive tract, lungs, liver and pancreas Mesoderm “Means”oderm
Muscles, skeleton, circulatory system, gonads, kidney
I Rest All gates closed II Depolarization Na gates open+ III Repolarization Na gates inactivate+ K gates open+ IV Hyperpolarization All gates closed
Blood type
RBC antigen
Antibodies Donates to:
Receives From: A A anti-B A, AB A, O B B anti-A B, AB B, O AB A, B None AB only All O None anti-A, B All O only
Enzyme Production Site Function Site Function Pepsin Gastric glands(chief cells) Stomach Hydrolyzes specific peptide bonds
Trypsin Pancreas Small Intestine
Hydrolyzes specific peptide bonds Converts chymotrypsinogen to chymotrypsin Chymotrypsin Hydrolyzes specific peptide bonds Carboxypeptidases A and B Hydrolyzes terminal peptide bond at C-terminus Aminopeptidase Intestinal glands
Hydrolyzes terminal peptide bond at N-terminus Dipeptidases Hydrolyzes pairs of amino acids Enteropeptidase Converts trypsinogen to trypsin
Table 1
Hormone Source Action F ollicle-stimulating (FSH)
Anterior pituitary
Stimulates follicle maturation; spermatogenesis L uteinizing (LH) Stimulates ovulation; testosterone synthesis A drenocorticotropic (ACTH) Stimulates adrenal cortex to make and secrete glucocorticoids T hyroid-stimulating (TSH) Stimulates the thyroid to produce thyroid hormones P rolactin Stimulates milk production and secretion E ndorphins Inhibits the perception of pain in the brain G rowth hormone Stimulates bone and muscle growth/lipolysis Oxytocin Hypothalamus; stored in posterior pituitary
Stimulates uterine contractions during labor, milk secretion during lactation Antidiuretic (ADH, vasopressin) Stimulates water reabsorption in kidneys Thyroid hormones (T 3 , T 4 ) (^) Thyroid Stimulates metabolic activity Calcitonin Decreases (tones down) blood calcium level Parathyroid hormone Parathyroid Increases blood calcium level Glucocorticoids (^) Adrenal cortex Increases blood glucose level and decreasesprotein synthesis; anti-inflammatory Mineralocorticoids Increases water reabsorption in kidneys Epinephrine, Norepinephrine Adrenal medulla Increases blood glucose level and heart rate
Glucagon Pancreas
Stimulates conversion of glycogen to glucose in the liver; increases blood glucose Insulin (^) Lowers blood glucose; increases glycogen stores Somatostatin Supresses secretion of glucagon and insulin Testosterone Testes Maintains male secondary sexual characteristics Estrogen (^) Ovary/Placenta Maintains female secondary sexual characteristics Progesterone Promotes growth/maintenance of endometrium Melatonin Pineal Regulates sleep–wake cycles Atrial natriuretic peptide Heart Involved in osmoregulation and vasodilation Thymosin Thymus Stimulates T-cell development
Enzyme Production Site Function Site Salivary amylase (ptyalin) Salivary glands^ Mouth Pancreatic amylase Pancreas Small intestine Maltase Intestinal glands Small intestine Sucrase Intestinal glands Small intestine Lactase Intestinal glands Small intestine
Hydrolysis Reaction
Starch → maltose Starch → maltose Maltose → 2 glucoses Sucrose → glucose, fructose Lactose → glucose, galactose
Ectoderm “Attract”oderm
Nervous system, epidermis, lens of eye, inner ear Endoderm “Endernal” organs
Lining of digestive tract, lungs, liver and pancreas Mesoderm “Means”oderm
Muscles, skeleton, circulatory system, gonads, kidney
I Rest All gates closed II Depolarization Na gates open+ III Repolarization Na gates inactivate+ K gates open+ IV Hyperpolarization All gates closed
Blood type
RBC antigen
Antibodies Donates to:
Receives From: A A anti-B A, AB A, O B B anti-A B, AB B, O AB A, B None AB only All O None anti-A, B All O only
Enzyme Production Site Function Site Function Pepsin Gastric glands(chief cells) Stomach Hydrolyzes specific peptide bonds
Trypsin Pancreas Small Intestine
Hydrolyzes specific peptide bonds Converts chymotrypsinogen to chymotrypsin Chymotrypsin Hydrolyzes specific peptide bonds Carboxypeptidases A and B Hydrolyzes terminal peptide bond at C-terminus Aminopeptidase Intestinal glands
Hydrolyzes terminal peptide bond at N-terminus Dipeptidases Hydrolyzes pairs of amino acids Enteropeptidase Converts trypsinogen to trypsin
Hormone Source Action F ollicle-stimulating (FSH)
Anterior pituitary
Stimulates follicle maturation; spermatogenesis L uteinizing (LH) Stimulates ovulation; testosterone synthesis A drenocorticotropic (ACTH) Stimulates adrenal cortex to make and secrete glucocorticoids T hyroid-stimulating (TSH) Stimulates the thyroid to produce thyroid hormones P rolactin Stimulates milk production and secretion E ndorphins Inhibits the perception of pain in the brain G rowth hormone Stimulates bone and muscle growth/lipolysis Oxytocin Hypothalamus; stored in posterior pituitary
Stimulates uterine contractions during labor, milk secretion during lactation Antidiuretic (ADH, vasopressin) Stimulates water reabsorption in kidneys Thyroid hormones (T 3 , T 4 ) (^) Thyroid Stimulates metabolic activity Calcitonin Decreases (tones down) blood calcium level Parathyroid hormone Parathyroid Increases blood calcium level Glucocorticoids (^) Adrenal cortex Increases blood glucose level and decreasesprotein synthesis; anti-inflammatory Mineralocorticoids Increases water reabsorption in kidneys Epinephrine, Norepinephrine Adrenal medulla Increases blood glucose level and heart rate
Glucagon Pancreas
Stimulates conversion of glycogen to glucose in the liver; increases blood glucose Insulin (^) Lowers blood glucose; increases glycogen stores Somatostatin Supresses secretion of glucagon and insulin Testosterone Testes Maintains male secondary sexual characteristics Estrogen (^) Ovary/Placenta Maintains female secondary sexual characteristics Progesterone Promotes growth/maintenance of endometrium Melatonin Pineal Regulates sleep–wake cycles Atrial natriuretic peptide Heart Involved in osmoregulation and vasodilation Thymosin Thymus Stimulates T-cell development
Enzyme Production Site Function Site Salivary amylase (ptyalin) Salivary glands^ Mouth Pancreatic amylase Pancreas Small intestine Maltase Intestinal glands Small intestine Sucrase Intestinal glands Small intestine Lactase Intestinal glands Small intestine
Hydrolysis Reaction
Starch → maltose Starch → maltose Maltose → 2 glucoses Sucrose → glucose, fructose Lactose → glucose, galactose
Regions of Electron Density Example
Geometric Arrangement of Electron Pairs around the Central Atom
Shape Angle betweenElectron Pairs
A Lewis acid–base adduct with a cation bonded to at least one electron pair donor (including water). Donor molecules are called ligands and use coordinate covalent bonds. The central cation can be bonded to the same ligand multiple times in a process called chelation.
δ+ δ+
δ–
δ+^ δ+
δ–
δ+ H (^) Cl
δ+
δ– δ+
H (^) Cl δ–
H (^) Cl δ–
δ+^ δ–
δ+^ δ–^ δ+^ δ–
nucleus
electron
symmetrical distribution
asymmetrical distribution
A mole is the amount of a substance that contains the same number of particles that are found in a 12.000 g sample of carbon-12. The molecular or formula weight is measured in amu per molecule (or formula unit). The molar mass is measured in grams per mole. Combustion reactions: A fuel, such as a hydrocarbon, is reacted with an oxidant, such as oxygen, to produce an oxide and water. CH 4 ( g ) + 2 O 2 ( g ) → CO 2 ( g ) + 2 H 2 O ( g )
Combination reactions: Two or more reactants form one product. S ( s ) + O 2 ( g ) → SO 2 ( g )
Decomposition reactions: A compound breaks down into two or more substances, usually as a result of heating or electrolysis. 2 HgO ( s ) → 2 Hg ( l ) + O 2 ( g ) Single-displacement reactions: An atom (or ion) of one compound is replaced by an atom of another element. Zn ( s ) + CuSO 4 ( aq ) → Cu ( s ) + ZnSO 4 ( aq ) Double-displacement reactions: Also called metathesis reactions; elements from two different compounds displace each other to form two new compounds. CaCl 2 ( aq ) + 2 AgNO 3 ( aq ) → Ca(NO 3 ) 2 ( aq ) + 2 AgCl ( s )
Net ionic equations: These types of equations are written showing only the species that actually participate in the reaction. Consider the following equation: Zn ( s ) + Cu 2+^ ( aq ) + SO 4 2–^ ( aq ) → Cu ( s ) + Zn2+^ ( aq ) + SO2– 4 ( aq ) The spectator ion (SO 4 2–) does not take part in the overall reaction, but simply remains in solution throughout. The net ionic equation would be: Zn ( s ) + Cu 2+^ ( aq ) → Cu ( s ) + Zn2+^ ( aq )
Neutralization reactions: These are a specific type of double-displacement reactions that occur when an acid reacts with a base to produce a solution of a salt (and, usually, water): HCl ( aq ) + NaOH ( aq ) → NaCl ( aq ) + H 2 O ( l ) Factors affecting reaction rates: reactant concentrations, temperature, medium, catalysts Catalysts are unique substances that increase reaction rate without being consumed; they do this by lowering the activation energy.
COMPOUNDS & STOICHIOMETRY
E auncatalyzed
reaction coordinate
free energy
E acatalyzed catalyzed
uncatalyzed
a A + b B ⇀↽ c C + d D
K c =
c d a b
K c is the equilibrium constant (c stands for concentration).
Pure solids and liquids don’t appear in expressions. K eq is characteristic of a given system at a given temperature. If K eq >> 1, an equilibrium mixture of reactants and products will contain very little of the reactants compared to the products. If K eq << 1, an equilibrium mixture of reactants and products will contain very little of the products compared to the reactants. If K eq is close to 1, an equilibrium mixture of products and reactants will contain approximately equal amounts of the two. Le Châtelier’s principle is used to determine the direction in which a reaction at equilibrium will proceed when subjected to a stress, such as a change in concentration, pressure, volume, or temperature. The key is to remember that a system to which these kinds of stresses are applied tends to change so as to relieve the applied stress.
In a nutshell:
n – 1
Experimental determination of rate law: The values of k , x , and y in the rate law equation (rate = k [A] x [B] y ) must be determined experimentally for a given reaction at a given temperature. The rate is usually measured as a function of the initial concentrations of the reactants, A and B.
E aforward
reaction coordinate
free energy Δ G
H 2 + Cl 2 E areverse
2 HCl
KINETICS & EQUILIBRIUM
The law of conservation of energy dictates that energy can be neither created nor destroyed, but that all thermal, chemical, potential, and kinetic energies are interconvertible.
Isolated: no exchange of energy/matter with the environment. Bomb calorimetry creates a nearly isolated system. Closed: can exchange energy but not matter with the environment Open: can exchange both energy and matter with the environment. Human beings are open systems because they can take in energy and matter (eat), release matter into the environment (respiration, urination, defecation), and release energy into the environment (heat transfer from the skin and mucous membranes).
THERMOCHEMISTRY
Osmotic pressure
∏ = M R T
Vapor pressure lowering (Raoult’s law)
P A = X A P A˚; P B = X B P ˚B
Solutions that obey Raoult’s Law are called ideal solutions.
heating of ice
heating of water
heating of water vapor
heat used to melt ice to water
heat used to vaporize water towater vapor
0
25
50
75
100
125
temperature (˚C)
heat added (each division = 4 kJ)
Graham’s law of diffusion and effusion
Diffusion: occurs when gas molecules distribute through a volume by random motion
Effusion: the flow of gas particles under pressure from one compartment to another through a small opening:
Both diffusion and effusion have the same formula: r r
1 2
1 2
m m 2 1
Percent composition by mass:
= Mass of solute× Mass of solution 100%
Mole fraction:
total # of moles in system
Molarity: # of mol of solute liter of solution
Molality: # of mol of solute kg of solvent
Normality:
liter of solution
SOLUTIONS
Arrhenius definition: An acid is a species that produces excess H +^ (protons) in an aqueous solution, and a base is a species that produces excess OH –^ (hydroxide ions). Brønsted–Lowry definition: An acid is a species that donates protons, while a base is a species that accepts protons. Lewis definition: An acid is an electron pair acceptor, and a base is an electron pair donor.
pH = –log[H+^ ] = log( 1 [H+]
pOH = –log[OH – ] = log( 1 [OH^ − ]
H 2 O ( l ) ⇀↽ ^ H +^ ( aq ) + OH–^ ( aq ) K w = [H+][OH–] = 10– pH + pOH = 14
HA ( aq ) + H 2 O ( l ) ↽ ⇀ ^ H 3 O+^ ( aq ) + A–^ ( aq )
K a =
K b =
Salt formation: Acids and bases may react with each other, forming a salt and (often, but not always) water in a neutralization reaction. HA + BOH → BA + H 2 O Hydrolysis: This is the reverse reaction, where the salt ions react with water to give back the acid and base. Amphoteric species: is one that can act either as an acid or a base, depending on its chemical environment
Titration and Buffers
strong acid and strong base
weak acid and strong base Henderson–Hasselbalch equation: is used to estimate the pH of a solution in the buffer region where the concentrations of the species and its conjugate are present in approximately equal concentrations
pH = p K a + log [conjugate base] [weak acid]
pOH = p K b + log [conjugate acid] [weak base]
ACIDS AND BASES
Titration is a procedure used to determine the molarity of an acid or base by reacting a known volume of a solution of unknown concentration with a known volume of a solution of known concentration. The half- equivalence point defines pH = p K a
B A S IC
A C ID IC
3 3
2 3 4 4
Oxidation: loss of electrons
Reduction: gain of electrons Oxidizing agent: causes another atom to undergo oxidation, and is itself reduced Reducing agent: causes another atom to be reduced, and is itself oxidized
A redox reaction occurring in a galvanic cell has a negative ∆ G and is therefore a spontaneous reaction. Galvanic cell reactions supply energy and are used to do work. This energy can be harnessed by placing the oxidation–reduction half-reactions in separate containers called half-cells. The half-cells are then connected by an apparatus that allows for the flow of electrons.
e
e
A redox reaction occurring in an electrolytic cell has a positive ∆ G and is therefore nonspontaneous. In electrolysis, electrical energy is required to induce a reaction. The oxidation and reduction half-reactions are usually placed in one container. Reduction potential of each species is defined as the tendency of a species to acquire electrons and be reduced. Standard reduction potential, E ˚, is measured under standard conditions: 25˚C, 1 M concentration for each ion in the reaction, a partial pressure of 1 atm for each gas and metals in their pure state. Standard reduction potentials are used to calculate the standard electromotive force (emf or E (^) cell˚ ) of a reaction, the difference in potential between two half-cells. emf = E red, cathode˚ – E red, anode˚
Gibbs free energy , ∆ G , is the thermodynamic criterion for determining the spontaneity of a reaction. ∆ G = – n F E cell
REDOX REACTIONS & ELECTROCHEMISTRY
Carboxylic acids have p K a values around 4.5 due to resonance stabilization of the conjugate base. Electronegative atoms increase acidity with inductive effects. Boiling point is higher than alcohols because of the ability to form two hydrogen bonds.
Oxidation of primary alcohols with KMnO (^4)
OH
O OH
KMnO (^4)
Hydrolysis of nitriles
CH 3 Cl CH 3 CN CH 3 COH
O
Formation of soap by reacting carboxylic acids with NaOH; arrange in micelles
O —Na +
O
nonpolar tail polar head
Nucleophilic acyl substitution
Decarboxylation O C O
CARbOXyLIC ACIDS Carboxylic acid derivatives contain three bonds to heteroatoms (O, N, halides, and so forth). As such, they can be interconverted through nucleophilic acyl substitution by swapping leaving groups. Carboxylic acid derivatives can be ranked based on descending reactivity:
Synthesis via dehydration of two carboxylic acids
Intramolecular formation of a cyclic anhydride
ortho -phthalic acid
OH OH
O
O
O
O
O
phthalic anhydride
CARbOXyLIC ACID DERIVATIVES
Cyclic amides are called lactams. These are named according to the carbon atom bound to the nitrogen: β-lactams contain a bond between the β-carbon and the nitrogen, γ-lactams contain a bond between the γ-carbon and the nitrogen, and so forth.
N H
O N H O N H O N H O β-lactam γ-lactam δ-lactam ε-lactam
Cyclic esters are called lactones. These are named not only based on the carbon bound to the oxygen, but also the length of the carbon chain itself.
O
O O
O O O O
O
α-acetolactone β-propiolactone γ-butyrolactone δ-valerolactone
CyCLIC CARbOXyLIC ACID DERIVATIVES
Reagents: aldehyde, ammonium chloride (NH 4 Cl), potassium cyanide (KCN) R O NH+ 3
NH 2 NH+ 2 OH+ 2
H NH+^3 O+
NH 3 R H
−CN −H 2 O
protontransfer
R (^) N
R OH
R
NH (^2) R
N (^) NH+ H+^ H2 O
transferproton H H2 N O
OH
R NH
OH+ 2
NH
OH+ 2
OH NH+ 2
proton transfer
−NH
OH OH NH+ 3
−H+
O OH
OH+ OH
H 2 N R
H2 N R
H 2 N R
H2 N R
H2N R
H 2 N R
H 2 N R
Reagents: potassium phthalimide, diethyl bromomalonate
O
S (^) N 2
O N K
phthalimidepotassium bromomalonatediethyl
CO 2 C 2 H 5 CO2C 2 H (^5)
CO2 C 2 H 5 CO2 C 2 H 5
base O
O CO 2 C 2 H 5 CO2C 2 H 5
R–Br
O
O −CO 2 C RH^3 O+,^ ∆ H
H 3 +NCO^2 H H 2 NCCO 2 R CO (^2) H^ NaOH 2 O, ∆ C O
O N R
CO 2 C 2 H (^5) CO 2 C 2 H 5
O
O N
O
O
−
−
−
−
−
−
(^) Br C H C H N C SN 2
NITROGEN-CONTAINING COMPOUNDS
PHOSPHORUS-CONTAINING COMPOUNDS
Formation from an anhydride
O – O
O
H 2 N (^) H
O – O
O
H 3 N
O OH
O
O O NH 3 +
O NH (^2)
Formation from an ester
Hydrolysis (requires acid)
Reduction to an amine O
NH (^2)
LiAlH 4 NH (^2)
Transesterification
Hydrolysis
H 2 O O
H3O+
+OH
OH O OH (^) H
OH OH
O + OH
OH O H O+H
H 2 O
Reduction
O
O LAH (^) OH + OH
Saponification
O
O
O
RC
RC
RC
O
O
O
RC O Na+
O
RC O Na+
O
RC O Na+
O
NaOH + HO HO
HO
triacylglycerol soap glycerol
Type of Chromatography Mobile Phase Stationary Phase Common Use Thin-layer or Paper Nonpolar solvent Polar card Identify a sample Reverse-phase Polar solvent Nonpolar card Identify a sample
Column Nonpolar solvent zPolar gel or powder Separate a sample into components Ion-exchange Nonpolar solvent Charged beads in column Separate components by charge
Size-exclusion Nonpolar solvent Polar, porous beads in column Separate components by size
Affinity (^) Nonpolar solvent
Beads coated with antibody or receptor for a target molecule
Purify a molecule (usually a protein) of interest
Gas (GC) Inert gas Crushed metal or polymer Separate vaporizable compounds
High-performance liquid (HPLC) Nonpolar solvent Small column with concentration gradient
Similar to column, but more precise
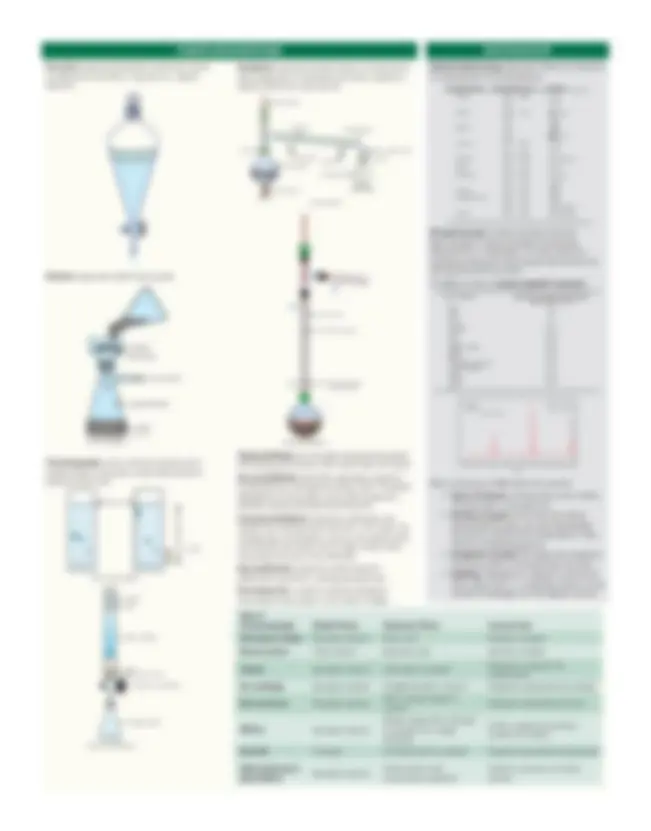
Extraction separates dissolved substances based on differential solubility in aqueous vs. organic solvents.
Filtration separates solids from liquids.
residue filter paper
to vacuum trap
clean filter flask
filtrate vacuum filtration
Chromatography uses a stationary phase and a mobile phase to separate compounds based on polarity and/or size.
thin-layer chromatograms
solvent front
Y
X
R f = (^) YX
11 22 33 11 22 333
solvent sand
sand
silica or alumina
glass wool or cotton stopcock to control flow
collection flask
column chromatography
PURIFICATION METHODS
Distillation separates liquids based on boiling point, which depends on intermolecular forces. Types are simple, fractional, and vacuum.
vacuum distillation
ice bath
vacuum adapter
to vacuum source
receiving flask
distilling flaskwater outlet water inlet
heat source
clamp clamp
condenser
thermometer
Column
fractional distillation
glass projection to hold up packing
Column packing
Simple distillation can be used to separate two liquids with boiling points below 150°C and at least 25°C apart. Vacuum distillation should be used when a liquid to be distilled has a boiling point above 150°C. To prevent degradation of the product, the incident pressure is lowered, thereby lowering the boiling point. Fractional distillation should be used when two liquids have boiling points less than 25°C apart. By introducing a fractionation column, the sample boils and refluxes back down over a larger surface area, improving the purity of the distillate. Recrystallization separates solids based on differential solubility in varying temperatures. Electrophoresis is used to separate biological macromolecules based on size and/or charge.
Infrared spectroscopy measures molecular vibrations of characteristic functional groups.
Alkanes 2800 — 3000 (^) C H 1200 C C Alkenes 3080 — 3140 C H (^1645) C C Alkynes (^2200) C C 3300 C H Aromatic 2900 — 3100^ C H 1475 — 1625 C C Alcohols 3100 — 3500 (^) O H (broad) Ethers 1050 — 1150 (^) C O Aldehydes 2700 — 2900 (O)C H 1700 — 1750 C O Ketones 1700 — 1750 C O Carboxylic acids 1700 — 1750 (^) C O 2800 — 3200 (^) O H (broad) Amines 3100 — 3500 N H (sharp)
Functional Group Wavenumber (cm–1) Vibration
UV spectroscopy involves passing ultraviolet light through a chemical sample and plotting absorbance vs. wavelength. It is most useful for studying compounds containing double bonds and heteroatoms with lone pairs. (^1) H–NMR is a form of nuclear magnetic resonance. Type of Proton Approximate Chemical Shift (ppm) Downfield from TMS RC H 3 RC H 2 R 3 C H –C H =C H –C≡ C H Ar– H –C H X –C H OH/–C H OR RC H O RC H CO– –C H COOH/–C H COOR –CHO H– C H 2 O H ArO H –COO H –N H 2
4.6– 2– 6–8. 2–4. 3.4– 9– 2–2. 2–2. 1–5. 4– 10.5– 1–
TMS
shielding
deshielding
8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0. δ (ppm)
a
b (^) Cl C H (^) a Hb O C Hb Cl Hb
When analyzing an NMR spectrum, look for:
SPECTROSCOPy