







Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
An in-depth exploration of enzymatic kinetic resolution (ekr) and enantioselective enzymatic desymmetrization (eed) in the context of lipases in asymmetric synthesis. It covers the mechanisms, substrates, and examples of these reactions, as well as the calculation of the enantioselectivity factor. Additionally, it discusses the importance of acyl donors in acylation reactions and their role in achieving high yields.
Typology: Slides
1 / 12

This page cannot be seen from the preview
Don't miss anything!







1. EKR (enzymatic kinetic resolution)
racemic
fast reactingenantiomer
slow reactingenantiomer
enantiomer
enantiomer
2. EED (enantioseletive enzymatic desymmetrization)
Maximum 50% yield can be obtianed for individual enantiomers
hi^
l
pro-chiralX = O, NH, SH
enantiopure
meso
enantiopure
Maximum 100% yield can be obtained for individual enantiomers
3. Dynamic kinetic resolution (DKR)
(+)-product E^
*A and A' are enantiomers; racemization can be done enzymaticallyor by means of other methods.
(-)-product X
or by means of other methods.* Maximum 100% yield can be obtained for individual enantiomers
Acyl donors for acylation reactions
7
Acyl
donors for acylation reactions
3 7
3
15
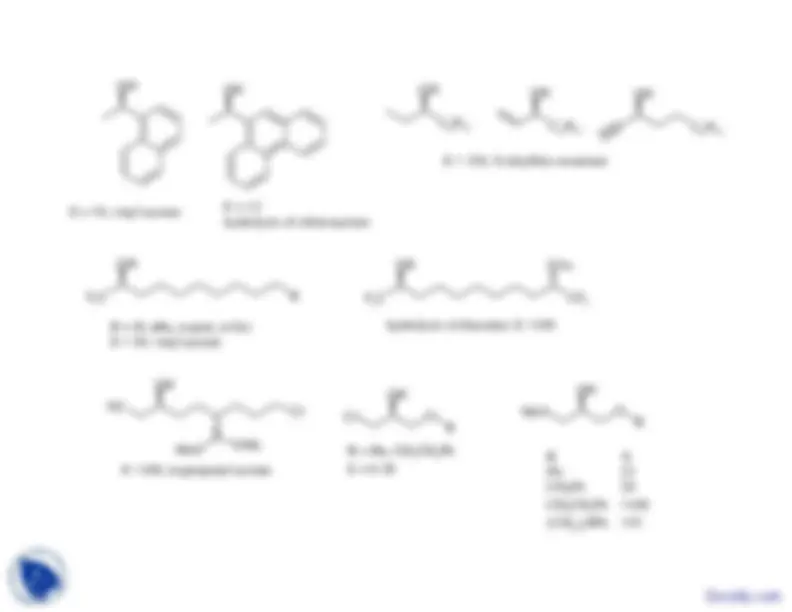
Selected examples of 2-alkanols resolved by CAL-B OH^
n
E >150, S-ethyl thiooctanoate
E >100, vinylacetate
n = 6, E >100S-methyl thioacetate
Bn^
Ph^
1-naphthyl 57
Br
1 naphthyl 572-naphthyl 66^ Diketene
E >100, S-ethyl thiooctanoate
73-99% ee for diacylated products
R = H, Br, CH
OTr, Ph 2
E >100; vinyl acetate
R = H, Br, CH
X = Cl, BrE >
13
13
13
E > 150, S-ethylthio octanoate
OAc
E = 10, vinyl acetate
E = 13hydrolysis of chloroacetate
OAc
R = H, nBu, n-pent, n-OctE = 50 vinyl acetate
hydrolysis of diacetate; E >
Cl^
Cl
MeO
50, vinyl acetate
OMe
MeO
E >100, isopropenyl acetate
R = Bn, CH
Ph 2
Ph^
Ph^2
Ph^2
)OPh 22
OH^
HN OH^
OH
Selected examples of cyclic 2-alkanols resolved by CRL OH^ R
N O MeO
C 2
OH
R = Et, C
H 6 13 , Ph
E >50hydrolysis of acetate
E = 10vinyl acetate
OH^
O OH
S S
OH R
OH OH Ph
hydrolysis of acetate
OBn
S
OH^
OH^
OH^
OH
E = 24vinyl acetate
E = 20-100; R = n-alkyl
CRL, E > 100vinyl acetate
CF^3 n
OH Ph^
Ph^
CH^5
11
SPh SPh
ON^2
OH
E >100, vinyl acetate
E >50vinyl acetate
E >100h d^
l^ i^
f^
t t
E = 11isopropenyl acetate
OAc CCl MeO
y^
hydrolysis of acetate substituents with similar sizes
CCl
3
MeOE > 50hydrolysis of acetate
substituents with similar sizes
OH^
OH H
OH H
OH
Br
OH
Selected exam ples of cyclic secondary alcohols resolved by C R L
H^
H
O
Ph Ph
NH O
R
E = 50, hydrolysisof butyrate
E = 27, hydrolysisof acetate
E = 125vinyl acetate
E = 61-64hydrolysis of butyrateR^
iP^ Ph
OH^
OH
Ar
OH
OtBu N
N^3
OAc
Br
OAc
Br
vinyl acetate
R = iPr, Ph
E^ 10 t
50
E = 50
N^3
Br OAc
Br OAc
OH
O
O
OH
N^
OH
E = 10 to 50transesterification orhydrolysis of acetate
E^
50 Ar = Ph, 4tBuPhtransesterification
E > 100hydrolysis of butyrate
regioselective hydrolysis of diacetate
O^
O N
F
O
N^3
CO^2
Me
R OH
E = 20
E = 39hydrolysis of acetate
CRL
E^
8 24
OH OAc
OH
O Me OTBS
OH
Ph
y^
y
E >50, hydrolysisof formate
CRL, E = 8-24R = OM e, Cl, Br, I, SM ehydrolysis of butanoate
OAc
OAc
O Me
OTBS
O
98% eehydrolysis of diacetate (EED)
ee = 98%hydrolysis of diacetate (EED)
E = 36-76vinyl acetate