
































Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
study report of hyperammonemia
Typology: Study Guides, Projects, Research
1 / 39

This page cannot be seen from the preview
Don't miss anything!
































October - 2019
blood. Increased entry of ammonia to the brain is a primary cause of neurologic disorders, such as congenital deficiencies of urea cycle enzymes, hepatic encephalopathies, Reye syndrome, several other metabolic disorders, and some toxic encephalopathies. [69]
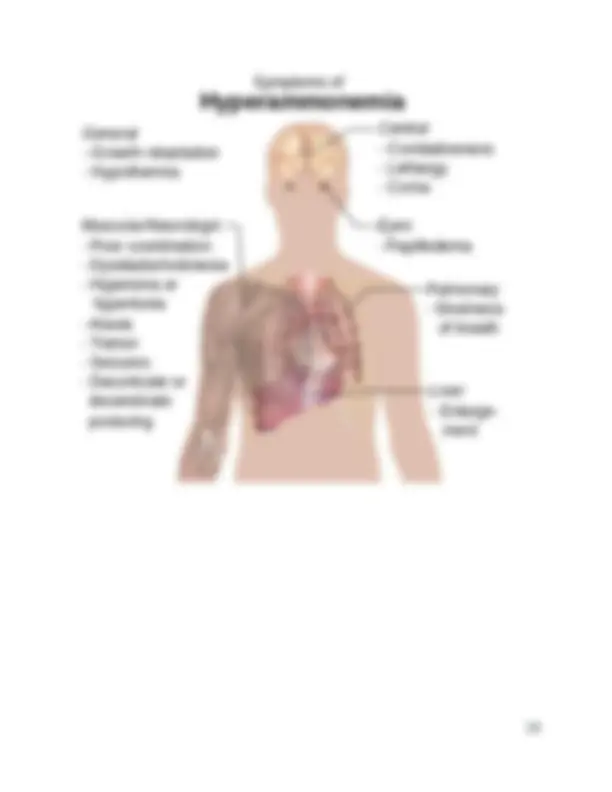
Signs and symptoms
Signs and symptoms of early-onset hyperammonemia (neonates) may include the following:
Diagnosis
No specific physical findings are associated with hyperammonemia. Affected infants usually present with the following:
Background Ammonia is a normal constituent of all body fluids. At physiologic pH, it exists mainly as ammonium ion. Reference serum levels are less than 35 μmol/L. Excess ammonia is excreted as urea, which is synthesized in the liver through the urea cycle. Sources of ammonia include bacterial hydrolysis of urea and other nitrogenous compounds in the intestine, the purine- nucleotide cycle and amino acid transamination in skeletal muscle, and other metabolic processes in the kidneys and liver. Increased entry of ammonia to the brain is a primary cause of neurological disorders associated with hyperammonemia, such as congenital deficiencies of urea cycle enzymes, hepatic encephalopathies, Reye syndrome, several other metabolic disorders, and some toxic encephalopathies.
Epidemiology United States Collecting accurate data on the frequency of metabolic disorders is difficult, because the information collected is representative of the particular area or the population group; the
incidence of urea cycle disorders in the United States is estimated at 1 in 25,000 live births.
International The prevalence of urea cycle disorders is currently estimated at 1:8,000-1:44,000 births internationally. The prevalence may be underestimated due to under diagnosis of fatal cases and unreliable newborn screening.
Mortality/Morbidity
Coma and cerebral edema are the major causes of death; the survivors of coma have a high incidence of intellectual impairment.
Race
These disorders have been observed in all races.
Sex
All the urea cycle disorders are inherited in an autosomal recessive pattern, except ornithine transcarbamoylase (OTC) deficiency, which is inherited as an X-linked trait; however, female carriers of the OTC gene can become symptomatic.
Age
Early-onset hyperammonemia presents in the neonatal period. Urea cycle disorders can present later in life.
Ammonia can be produced by the break-down of amino acids, or by the gut bacteria in humans. If thelevel of ammonia in the blood becomes too high, then it becomes toxic to the brain.The urea cycle removes ammonia from the blood and makes urea, which is eventually excreted as urine. This cycle is
determines how fast the cycle progresses. N-acetylglucosamine is also required for CPS to function, and functions as a regulator for the formation of urea.
Ornithine transcarbamoylase (OTC) then condenses carbamoyl phosphate and ornithine, which forms citrulline. This citrulline is then moved out of the mitochondria into the cytosol of the cell by the transporter SLC25A15.
The Cytosolic Stage
Argininosuccinate synthetase (AS) takes the citrulline formed in the mitochondrial stage, and condenses it with aspartate to form argininosuccinate. This occurs by the formation of an intermediate, citrulline-AMP.
Argininosuccinate is then broken into arginine and fumarate by argininosuccinate lyase (AL). Fumarate is then incorporated into another metabolic cycle, the TCA cycle. The TCA cycle can then reform aspartate, which is used by AS.
Arginine is then further broken down into urea and ornithine by arginase. Arginine can also be acquired from the diet, and this can also be taken in by the liver cells and broken down into urea and ornithine by arginase.
The ornithine is then transported into the mitochondria by ornithine translocase. There, it is used by OTC again, to form citrulline. The citrulline is then processed to form urea and ornithine again, and the cycle continues. During the cycle, urea is the only new product which is formed, while all other molecules used in the cycle are recycled.
Ammonia is a product of the metabolism of proteins and other compounds, and it is required for the synthesis of essential cellular compounds. However, a 5- to 10-fold increase in ammonia in the blood induces toxic effects in most animal species, with alterations in the function of the central nervous system. Both acute and chronic hyperammonemia result in alterations of the neurotransmitter system. Based on animal study findings, the mechanism of ammonia neurotoxicity at the molecular level has been proposed. Acute ammonia intoxication in an animal model leads to increased extracellular concentration of glutamate in the brain and results in activation of the N -methyl D-aspartate (NMDA) receptor. Activation of this receptor mediates ATP depletion and ammonia toxicity; sustained blocking of the NMDA receptor by continuous administration of antagonists dizocilpine (MK-801) or memantine prevents both phenomena, leading to significantly increased survival time in rats. [4]^ The
ATP depletion is due to activation of Na+^ /K+^ -ATPase, which, in turn, is a consequence
of decreased phosphorylation by protein kinase C. Activation of the NMDA receptor is probably the cause of seizures in acute hyperammonemia. Neuropathologic evaluation demonstrates alteration in the astrocyte morphology. Studies demonstrated a significant downregulation of the gap–junction channel connexin 43, the water channel aquaporin 4 genes, and the astrocytic inward-rectifying potassium channel genes, colocalized to astrocytic end-feet at the brain vasculature, where they regulate potassium and water transport. A downregulation of these channels in hyperammonemic mice suggests an alteration in astrocyte-mediated water and
potassium homeostasis in the brain as a potential key factor in the development of brain edema. [5] Also, studies on cultured astrocytes examined the potential role of p53, a tumor suppressor protein and a transcriptional factor, in ammonia-induced neurotoxicity. Activation of p53 contributes to astrocyte swelling and glutamate uptake inhibition, leading to brain edema. Both processes are blocked by p53 inhibition. [6] High levels of ammonia induce other metabolic changes that are not mediated by activation of the NMDA receptor and thus are not involved directly in ammonia-induced ATP depletion or neurotoxicity. These include increases in brain levels of lactate, pyruvate, glutamine, and free glucose, and decreases in brain levels of glycogen, ketone bodies, and glutamate. Chronic hyperammonemia is associated with an increase in inhibitory neurotransmission as a consequence of 2 factors. The first is down regulation of glutamate receptors secondary to excessive extra synaptic accumulation of glutamate. In addition, changes in the glutamate-nitric oxide-cGMP pathway result in impairment of signal transduction associated with NMDA receptors, leading to alteration in cognition and learning. [7]^ The second is an increased GABA ergic tone resulting from
benzodiazepine receptor overstimulation by endogenous benzodiazepines and neurosteroids. These changes probably contribute to deterioration of intellectual function, decreased consciousness, and coma. Treatment of chronic hyperammonemic rats with inhibitors of phosphodiesterase 5 restores the function of glutamate-nitric oxide-cGMP pathway and cGMP levels in rats’ brain, with restored ability to learn a conditional task. [8]
History
Family history may reveal unexplained neonatal deaths or undiagnosed chronic illness. A history of males being affected is suggestive of OTC deficiency, which is inherited as an X-linked trait. Consanguinity results in an increased risk of inheriting a metabolic disorder. Early-onset hyperammonemia presents in the neonatal period. The baby is usually well for the first day or two. As the ammonia level rises, the baby becomes symptomatic. The family gives a history of lethargy, irritability, poor feeding, and vomiting. These symptoms correlate with an ammonia level of 100-150 μmol/L, which is 2-3 times the reference range. This may be followed by hyperventilation and grunting respiration; seizures also may develop. Late-onset hyperammonemia typically is due to urea cycle disorders, which can present later in life. The frequently altered clinical presentation of urea cycle disorders later in life develops from intrinsic differences in physiology based on age, as well as molecular aspects of the underlying biochemistry. Older children have greater energy reserves than neonates, allowing them to compensate for periods of stress. They also have a greater capacity and more opportunity to regulate their own environment. Adults with partial enzyme deficiency can become symptomatic when hyperammonemia is triggered by a stressful medical condition such as postpartum stress, heart-lung transplant, short bowel and kidney disease, parenteral nutrition with high nitrogen intake, and gastrointestinal bleeding.
Physical
Enzyme defects in urea cycle
See the list below:
60% of OTC deficiency diagnoses are made in non-neonates. The oldest reported patient was aged 61 years.
Organic acidemias