





Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
This lecture is part of lecture series on Enzymes in Functional Group Transformation course. This lecture was delivered in Biochemistry class at Deenbandhu Chhotu Ram University of Science and Technology. This lecture main points are: Enzymes, Transformation, Oxidoreductases, Transferases, Lyases, Isomerases, Ligases, Alcohols
Typology: Slides
1 / 12

This page cannot be seen from the preview
Don't miss anything!







1 Docsity.com
1. Oxidoreductases
Oxidation-reduction reactions
2. Transferases
Transfer of functional groups
3. Hydrolases
Hydrolysis reactions
3.
yd o ases
yd o ys s
eact o s
4. Lyases
Group elimination (forming new bonds)
5 Isomerases
Isomerization reactions
5. Isomerases
Isomerization reactions
6. Ligases
Bond formation coupled withatriphosphate cleavage
In
the
subsequent
section
we
will
discuss
the
synthetic
potentials
of
oxidoreductases, hydrolases, lyases and isomerases due to their wide applicabilityin organic synthesis.
Docsity.com
Docsity.com
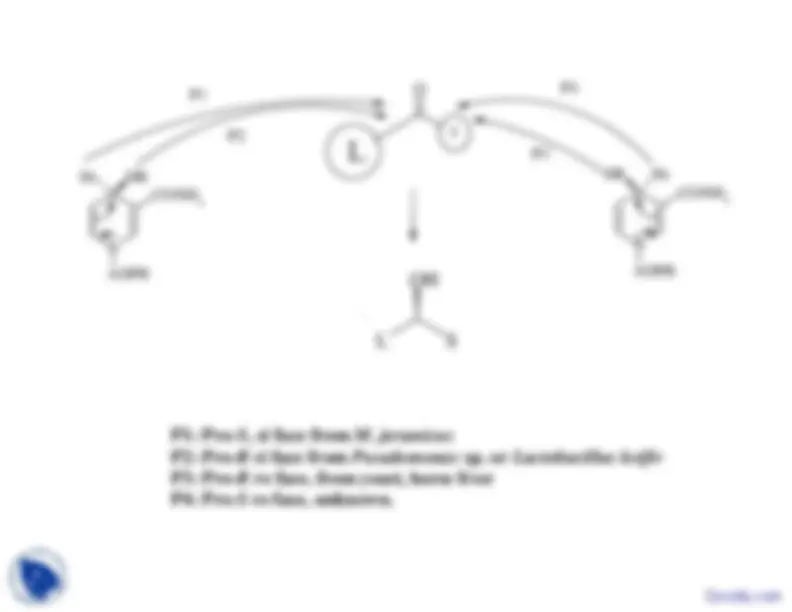
O
P
P
CONH HR
Hs
CONH Hs
HR
L
S
P
P
N
CONH
2
N ADPR
CONH
2
ADPR
ADPR
Docsity.com
a^
b
Enzyme 1
Enzyme 1
a.^
b.
+^
2
Enzyme 2
Auxiliary substrate
Enzyme 2
Auxiliary substrate
Auxiliary substrate
Docsity.com
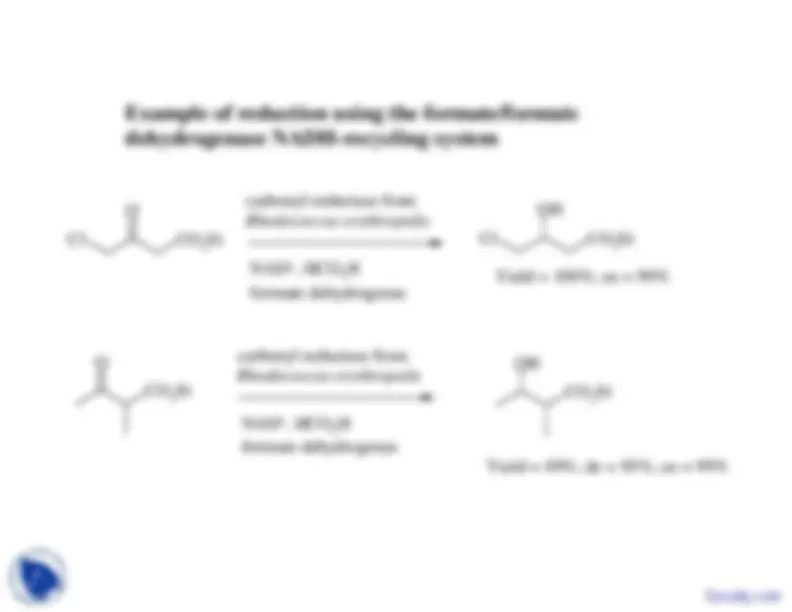
Cl
Et 2
Cl
Et^2
carbonyl reductase from Rhodococcus erythropolis^ NAD
+^
formate dehydrogenas
Yield = 100%; ee = 99%
Et 2
Et 2
carbonyl reductase from Rhodococcus erythropolis^ NAD
+^
formate dehydrogenas
Yield = 49%; de = 95%; ee = 99%
Docsity.com
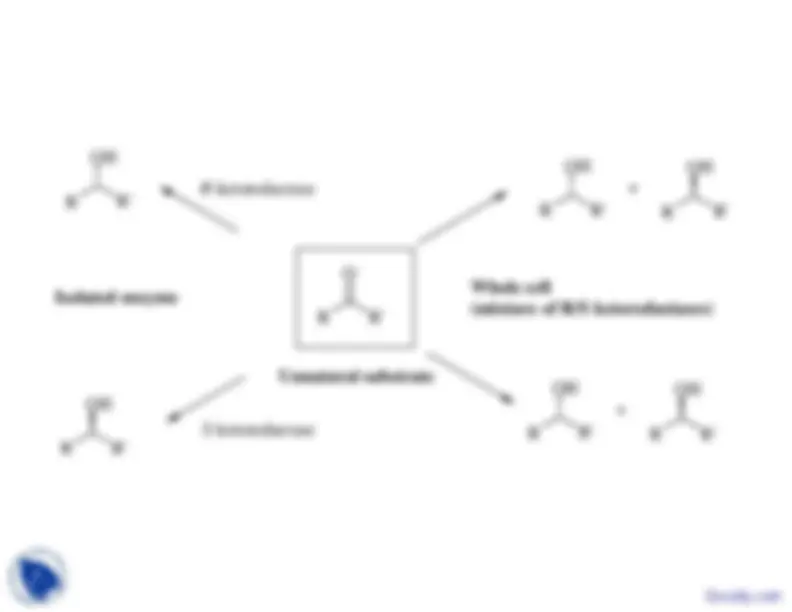
R -ketoreductase
R -ketoreductase
Isolated enzyme
Whole cell(mixture of R/S ketoreductases)
Unnatural substrate
S -ketoreductase
Docsity.com
Synthesis of both enantiomers using one microorganism by choosing appropriate conditions
R^
R' O
R^
R' OH
R^
R' OH
inhibitor of R-enzymeor activator of S-enzyme
inhibitor of S-enzymeor activator of R-enzyme
R^
R
Unnatural substrate
(S)
(R) Docsity.com