













Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
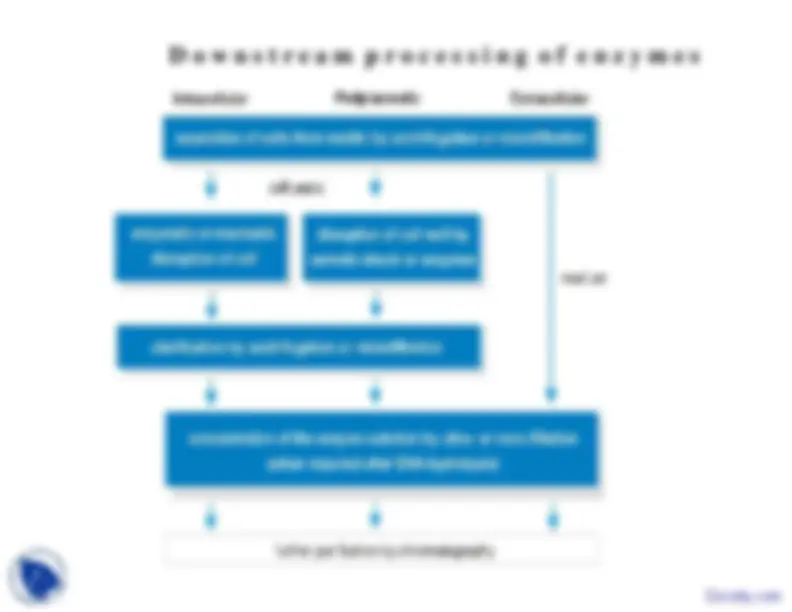
This lecture is part of lecture series on Enzymes in Functional Group Transformation course. This lecture was delivered in Biochemistry class at Deenbandhu Chhotu Ram University of Science and Technology. This lecture main points are: Enzyme, Characterization, Purification, Protein, Modulating, Solubility, Precipitation, Salting In, Ammonium
Typology: Slides
1 / 25

This page cannot be seen from the preview
Don't miss anything!


















p^ p
occur, and then centrifuge the solution to remove theprecipitate.• You take the supernatant, and add ammonium sulfate untilit reaches a concentration of 40%, allow precipitation tod th
t if th^
l ti^ t^
th
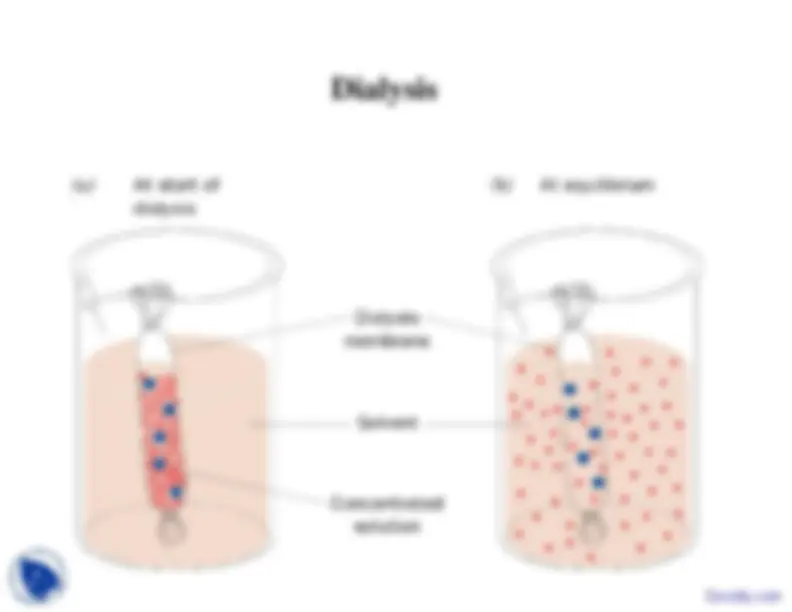
occur, and then centrifuge the solution to remove theprecipitate.• You dissolve the precipitate in a buffer and dialyze it toremove the salt.
(a.k.a. "molecular sieve", "size exclusion")• The protein is applied to the top of a column consisting ofporous beads made of a hydrated material, such as agarose,dextran, or polyacrylamide.,^
p^ y^ y
h^
i^
f^ i^
d^ i
is the volume surrounding the beads.
is the total volume of the column.
/3b d V^ is typically about Vvoid^
/3bed^
l^
f^ l^ t^
i^ d t^ l t
it f^ th
which is the volume of solvent required to elute it from thecolumn. The relative elution volume (=V
/ V^ elution void ) is a quantity
independent of the particular column used.• By standardizing the column with proteins of known size, onecan use this technique to estimate molecular weight (assuming thatcan use this technique to estimate molecular weight (assuming thatthe shape is close to that of the standards – more or less globular).
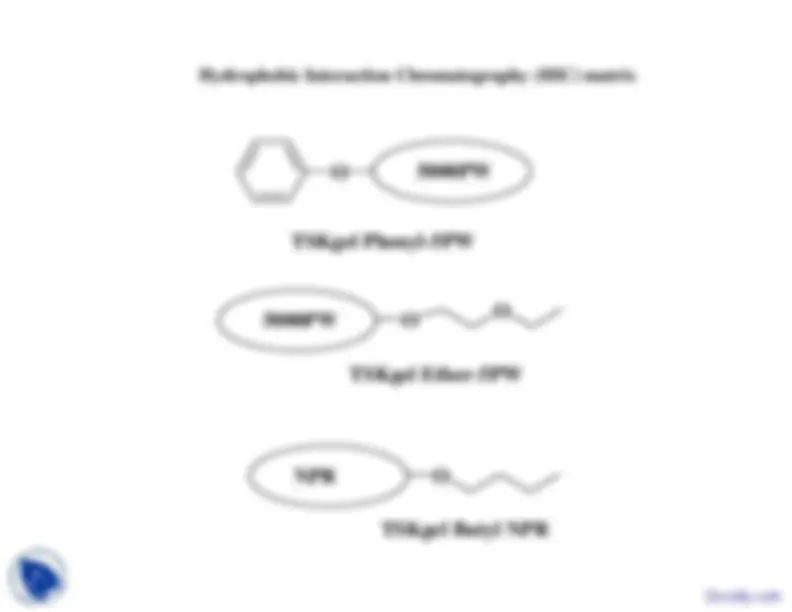
Commonly Used Gel Filtration Materials
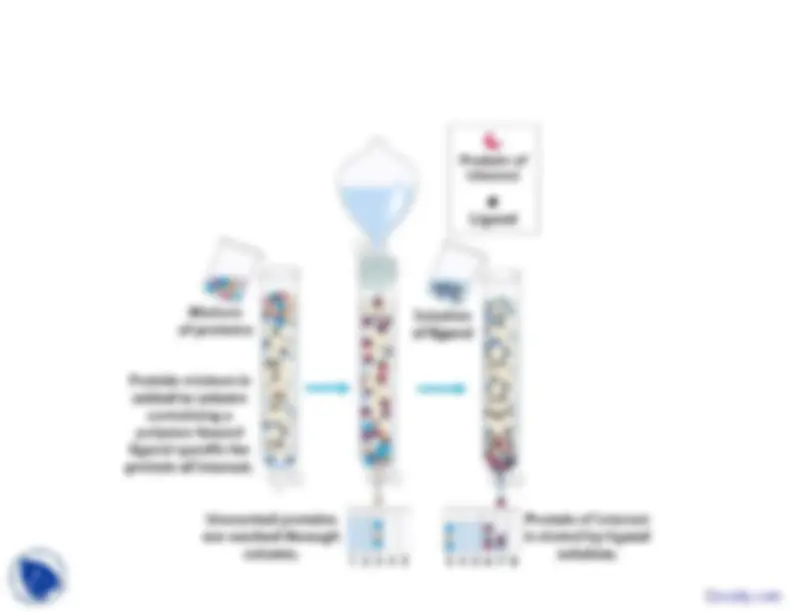
g^ p
opposite charge or low charge.• The protein can be eluted from the column either by changing• The protein can be eluted from the column either by changingthe pH or by increasing the salt concentration, which shieldsthe charges and thus decreases their attraction. Elution is mostf^
i d^ b^
l i^
l^ di^
h^ l
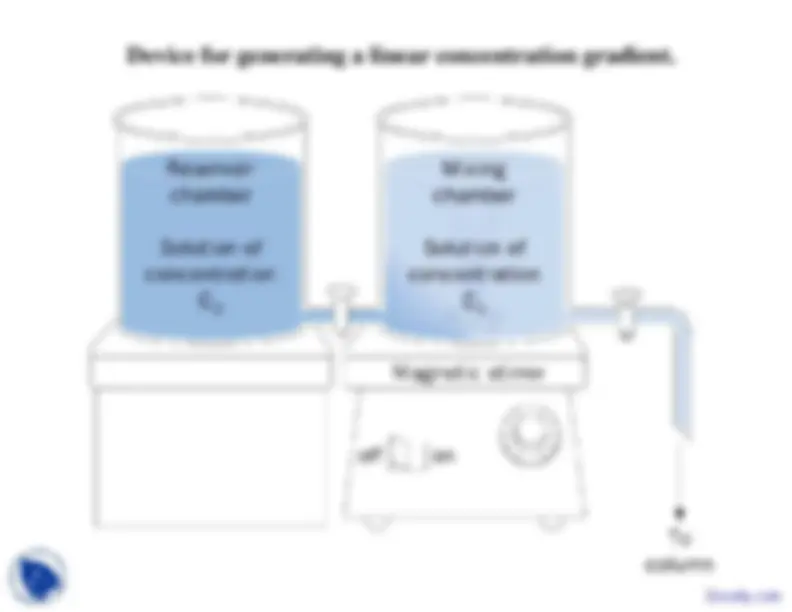
often carried out by applying a salt gradient to the column.
Device for generating a linear concentration gradient.
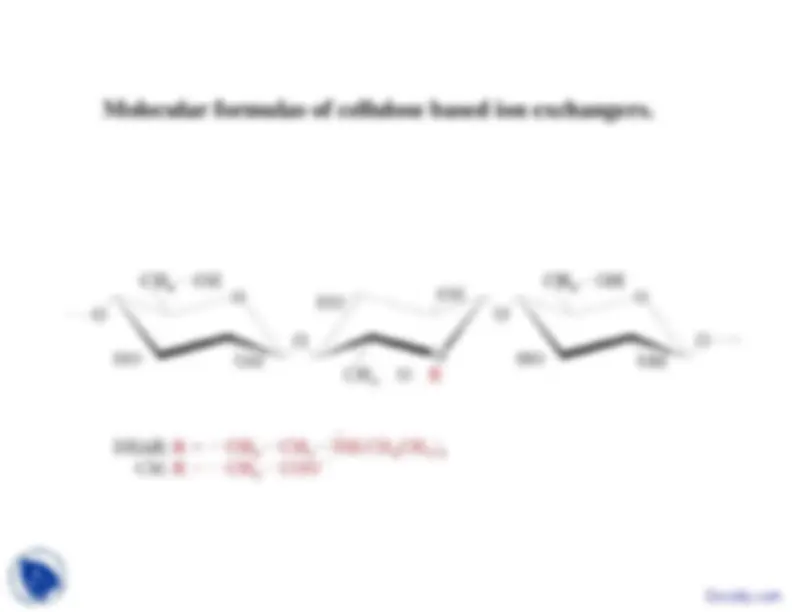
Molecular formulas of cellulose based ion exchangersMolecular
formulas of cellulose based ion exchangers.